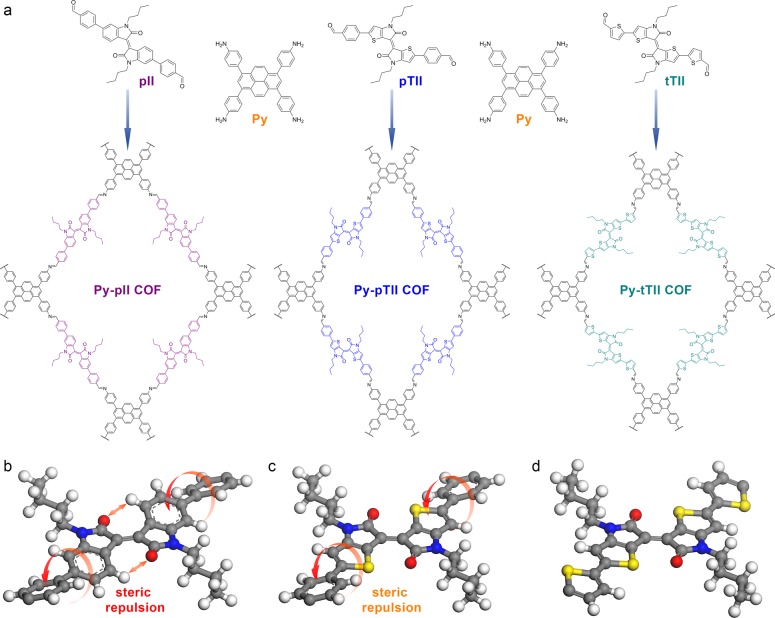
Figure 1.
(a) Co-condensation of 1,3,6,8-tetrakis(4-aminophenyl)pyrene (Py) with 2 equiv of the (thieno-)isoindigo-bridged dialdehydes pII, pTII, and tTII leads to the formation of the respective imine-linked 2D COFs. (b) The cut-out of the simulated Py-pII COF structure reveals that the pII building block is not entirely flat. Steric repulsion causes a rotation of the terminal phenyls vs the isoindigo core. Additionally, the repulsion between the ketones and adjacent hydrogen atoms leads to a slight distortion of the core itself. (c) These steric constraints are considerably relaxed when the benzene rings are replaced by thiophenes, resulting in a planar thienoisoindigo core. While the terminal phenyl rings remain slightly rotated vs the core in the case of pTII, exchanging them for thiophenes (d) yields the virtually planar tTII building block.