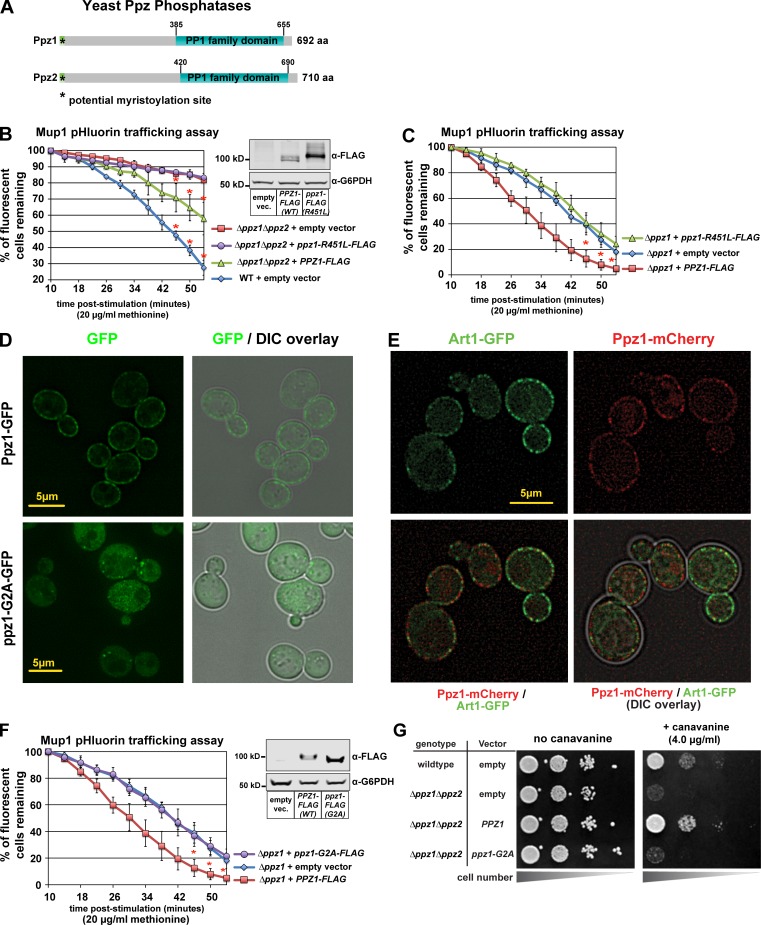
Figure 3.
Ppz catalytic activity and PM localization are required for Mup1 endocytosis. (A) Schematic representation of functional features known in Ppz1 and Ppz2 phosphatases. (B) Complementation analysis of Δppz1Δppz2 mutant yeast cells using the Mup1-pHluorin–trafficking assay. Error bars indicate standard deviation from multiple biological replicate experiments (n = 3). Expression of WT and catalytic dead (R451L) Ppz1-FLAG was confirmed by immunoblot (inset). (C) Complementation analysis of Δppz1 mutant yeast cells using the Mup1-pHluorin–trafficking assay. Error bars indicate standard deviation from multiple biological replicate experiments (n = 4). (D) Yeast cells expression WT Ppz1-GFP or a ppz1-G2A-GFP mutant were analyzed by fluorescence microscopy. (E) WT (strain SEY6210) yeast cells expressing Art1-GFP and Ppz1-mCherry were stimulated with methionine for 3 min and imaged using fluorescence deconvolution microscopy. (F) Complementation analysis of Δppz1 mutant yeast cells using the Mup1-pHluorin–trafficking assay. Immunoblot (inset) shows expression of Ppz-FLAG from indicated vectors. For all Mup1-pHluorin–trafficking assays, plots represents average of multiple biological replicate experiments (n ≥ 3), and error bars indicate standard deviation. For the final three time points of each time course, statistical significance was calculated using a Student’s t test (P < 0.005). (G) WT or Δppz1Δppz2 mutant yeast cells (SEY6210 strain background) harboring the indicated Ppz1 expression plasmids were plated on minimal media in the presence of canavanine (toxic arginine analogue).