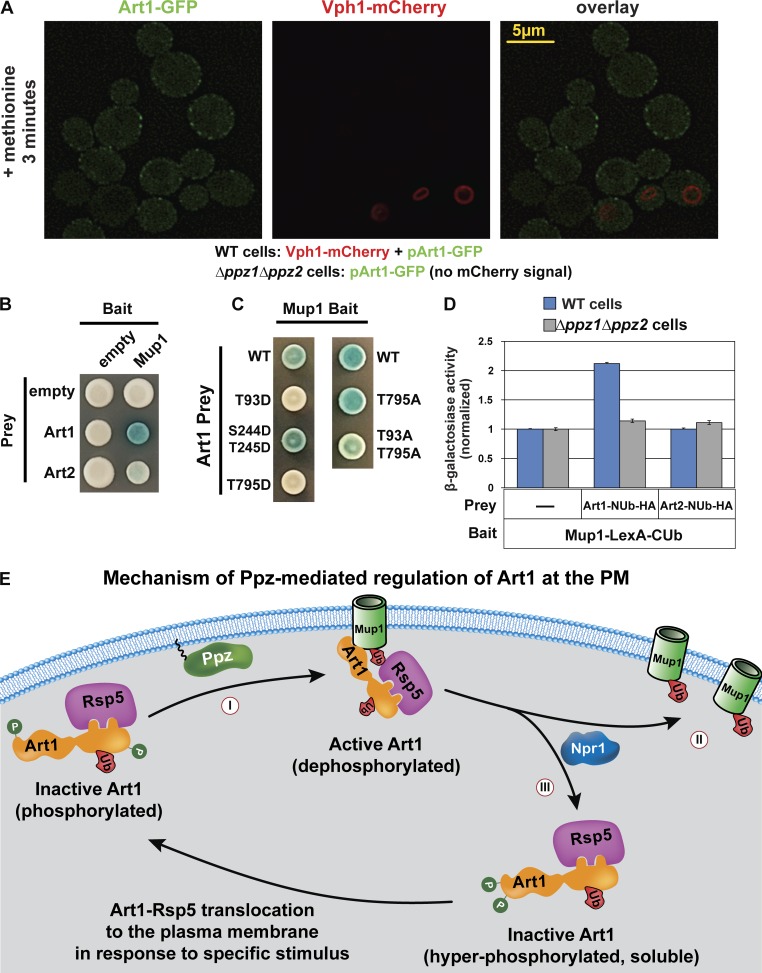
Figure 7.
Ppz phosphatases promote Art1 interaction with Mup1 at the PM. (A) WT yeast cells (expressing Vph1-mCherry) and Δppz1Δppz2 yeast cells (lacking mCherry expression) were grown to mid-log phase, mixed, stimulated with methionine for 3 min, and imaged by fluorescence deconvolution microscopy. (B and C) A split-ubiquitin two-hybrid system was used to probe for Art1-cargo interactions. Different cargo (Mup1, Can1, and Lyp1) were expressed as bait constructs fused to LexA and CUb. Art1 was expressed as a prey construct fused to NUb-HA. (D) β-Galactosidase activity assays were performed to quantify the Art1-Mup1 interaction in WT and Δppz1Δppz2 yeast cells. Error bars indicate standard deviation from multiple biological replicate experiments (n = 7). Expression of bait and prey constructs is shown in Fig. S5 (E and F). (E) An integrated model summarizing current understanding of Art1 phosphoinhibition at the PM. Ppz phosphatases promote dephosphorylation of Art1 at Thr245, Thr795, and Thr93 positions, which contributes to Art1 activation at the PM (I). Specifically, Ppz phosphatase activity promotes the interaction of Art1 with Mup1 at the PM. Activated Art1 targets Rsp5 activity to Mup1, which is ubiquitylated (II) and subsequently sorted for endocytosis. At the PM, Npr1 kinase antagonizes Art1 activity by phosphorylating its N terminus, resulting in Art1 departure from the PM (III); thus, both Ppz phosphatases and Npr1 kinase participate in a complex phosphorylation/dephosphorylation cycle that controls Art1 activity and cargo ubiquitylation.