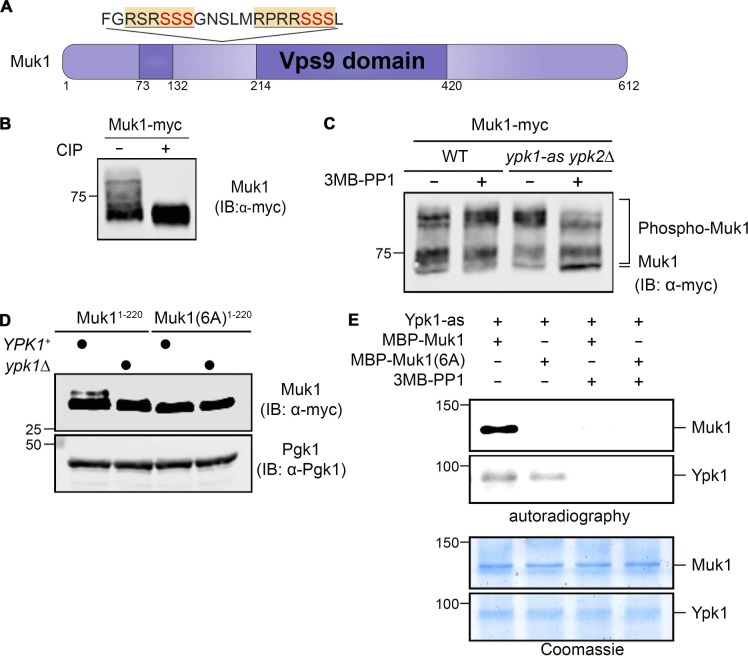
Figure 1.
Muk1 is phosphorylated by Ypk1 in vivo and in vitro. (A) Schematic depiction of Muk1. Dark purple, split catalytic (Vps9 homology) domain; yellow and underlined, consensus Ypk1 phospho-acceptor site; red, phosphorylated residues. (B) WT cells (BY4741) expressing Muk1-myc from the GAL promoter on a multi-copy (2 µm DNA) vector (pMLT22) were grown to mid-exponential phase, harvested, and lysed, and equivalent samples of the resulting extract protein were incubated in the absence (−) or presence (+) of CIP and, after treatment, resolved by SDS-PAGE in an 8% acrylamide gel and analyzed by immunoblotting (IB), all as described in Materials and methods. (C) WT (BY4741) or otherwise isogenic ypk1-as ypk2Δ (yAM123-A) cells expressing Muk1-myc as in B were grown to mid-exponential phase, treated with vehicle (DMSO) or 3MB-PP1 (10 µM final concentration) in the same solvent for 90 min, harvested, lysed, and examined as in B, except SDS-PAGE was conducted using a 7% acrylamide gel. (D) WT (BY4741) or otherwise isogenic ypk1Δ (JTY6142) cells expressing from the same vector as in B either Muk1(1–220; pMLT56) or Muk1(1–220 6A; pMLT57) were grown to mid-exponential phase, treated with AbA (1.8 µM final concentration) for 2 h, harvested, and lysed, and the resulting extracts were analyzed as in B, except SDS-PAGE was conducted using a 13.5% acrylamide gel. Pgk1 served as a loading control. (E) Equivalent amounts (∼0.5 µg) of MBP-Muk1-(His)6 or MBP-Muk16A-(His)6, expressed in and purified from E. coli, were incubated with [γ-32P]ATP and Ypk1-as purified from S. cerevisiae in the absence (–) or presence (+) of 3MB-PP1, as described in Materials and methods, and the resulting products were analyzed by both autoradiography (top) and staining with Coomassie Blue dye (bottom).