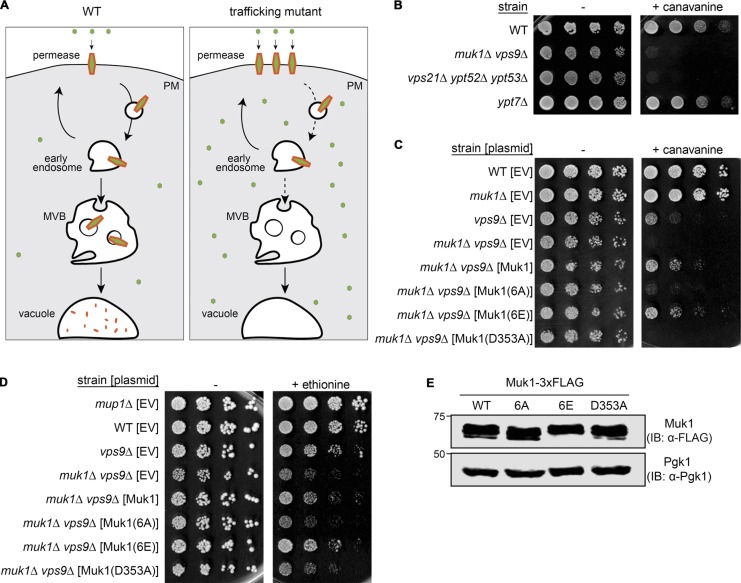
Figure 2.
Ypk1-dependent phosphorylation of Muk1 promotes endocytosis of permeases. (A) Schematic depiction of how inefficient internalization of amino acid permeases that reside in the PM results in increased sensitivity to the growth-inhibitory effect of a toxic amino acid analogue (small green circle). (B) Samples of exponentially growing cultures of otherwise isogenic strains of the indicated genotype were plated in fivefold serial dilutions on growth medium lacking Arg in the absence (–) or presence (+) of canavanine (5.7 µM final concentration). (C) Strains of the indicated genotype carrying an empty vector (EV; pRS315) or expressing from the same vector either Muk1-3xFLAG (pMLT83) or the indicated Muk1 variants Muk1(6A)-3xFLAG (pMLT84), Muk1(6E)-3xFLAG (pMLT92), or catalytically inactive Muk1(D353A)-3xFLAG (pMLT85), were grown and plated as in B. (D) Strains of the indicated genotype, as in C, were plated in fivefold serial dilutions on growth medium lacking Met in the absence (−) or presence (+) of ethionine (10 µM final concentration). (E) To confirm equivalent levels of expression, WT cells (BY4741) expressing the indicated Muk1-3xFLAG constructs, as in C and D, were grown to mid-exponential phase, harvested, and lysed, and proteins in the resulting extracts were treated with CIP, as in Materials and methods, resolved by SDS-PAGE on an 8% acrylamide gel, and analyzed by immunoblotting (IB).