Figure 1.
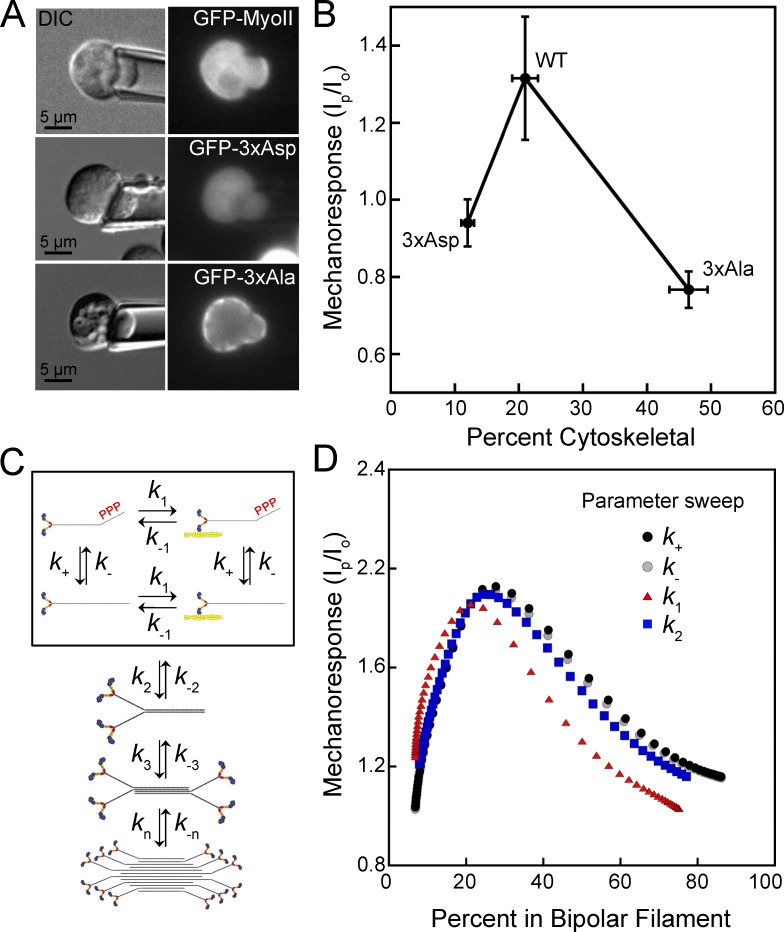
Myosin II cytoskeletal association and mechanoresponsiveness have a biphasic relationship in Dictyostelium. (A) GFP-MyoII accumulates to the region of the cell dilated by the pipette upon applied force. Mutant myosin II proteins mimicking heavy chain phosphorylation (GFP–3× Asp) or nonphosphorylatable mutants (GFP–3× Ala) do not respond to the applied force. (B) Mechanoresponsiveness suggests a biphasic dependency on the percentage of myosin that is cytoskeleton-associated. Peak myosin II mechanoresponse is calculated as the highest ratio of background-subtracted intensity in the pipette (Ip) to the background-subtracted intensity at the cortex at the opposite side of the cell (Io) in 5 min. Mechanoresponse values (y axis values) reproduced from Ren et al. (2009). Percent cytoskeletal is measured by cytoskeletal fraction and reproduced from Rai and Egelhoff (2011) with permission from the authors (x axis values). (C) Scheme to model the assembly of myosin II bipolar filaments, including exchange between assembly-incompetent, assembly-competent, actin-bound, and actin-unbound monomers (boxed). Monomers then assemble into dimers, tetramers, and bipolar filaments. (D) A computational model for myosin II mechanosensitive assembly predicts that when increased or decreased by an order of magnitude, rates affecting myosin II transition from assembly-incompetent to assembly-competent (k+ and k-), from actin unbound to actin bound (k1), or from monomer to dimer (k2) will reproduce a biphasic relationship between the percent of myosin II assembled and the mechanoresponse at 5 min.