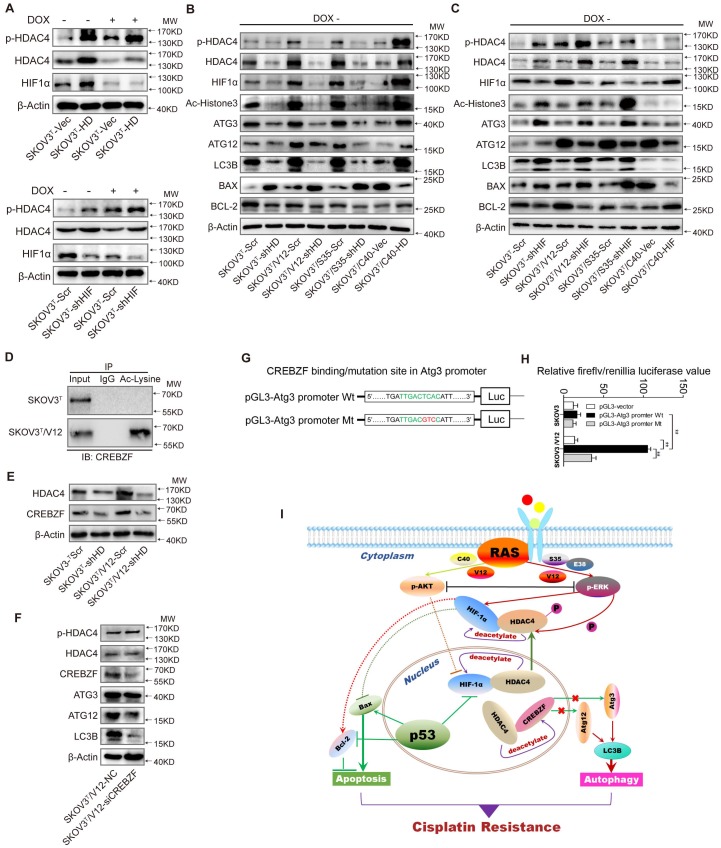
Figure 6.
HDAC4 modulated by p53 and HIF-1α regulates autophagy by deacetylating CREBZF. A. HDAC4 overexpression leads to increased HIF-1α stability and HDAC4 phosphorylation (upper panel), whereas knockdown of HIF-1α stimulates HDAC4 phosphorylation. B. Association of HDAC4 with autophagy and apoptosis. C. Association of HIF-1α with autophagy and apoptosis. D. Acetylation of CREBZF in SKOV3T and SKOV3T/V12 cells. E. CREBZF expression with HDAC4 knockdown in SKOV3T and SKOV3T /V12 cells. F. Atg3, Atg12 and LC3B expression with CREBZF knockdown. G. Construction of ATG3 promoter luciferase plasmids with the CREBZF binding/mutation sites. H. SKOV3T and SKOV3T /V12 cells were transfected with the plasmids of the ATG3 promoter-driven luciferase for 48 h followed by a dual luciferase assay. A high luciferase activity was observed in SKOV3T/V12 cells transfected with the wild type of Atg3 promoter, whereas the transfection of the same cell line with the binding site mutation promoter highly reduced the luciferase acitivity. **, P < 0.01. I. A schematic diagram showing that HDAC4 and HIF-1α are key mediators between p53 and RAS signaling networks although ERK and AKT inversely regulate HDAC4 and HIF-1α through phosphorylation, translocation and protein degradation. HDAC4 and HIF-1α collaboratively inhibit cellular apoptosis, but inversely control autophagy, both confer ovarian cancer cisplatin resistance. Protein markers are properly labeled in relative panels.