FIG 1.
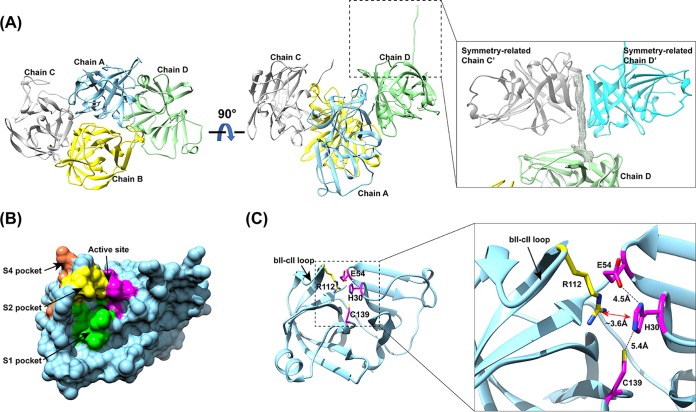
Structure of the HOV Pro. (A) Crystal packing of HOV Pro, with four molecules in the asymmetric unit shown in ribbon representation. The inset shows the C-terminal tail of chain D interacting with symmetry-related chains C′ and D′. The Fo-Fc difference electron density map of the C-terminal tail is shown in gray mesh and contoured at 3 σ. (B) Surface representation of the HOV Pro monomer with the color-coded active site (magenta), and S1 (green), S2 (yellow), and S4 (orange) substrate-binding pockets. (C) Cartoon representation of a protease monomer shown in the same orientation as in panel B. The inset shows a close view of the catalytic residues H30, E54, C139 (magenta), and R112 (yellow) of the S2 pocket rendered as sticks, with nitrogen shown in blue and oxygen shown in red. The distances between the residues are indicated by black dashed lines. The cation-π interaction between R112 and H30 is indicated by a red double arrow. The flexible bII-cII loop is indicated with black arrows.