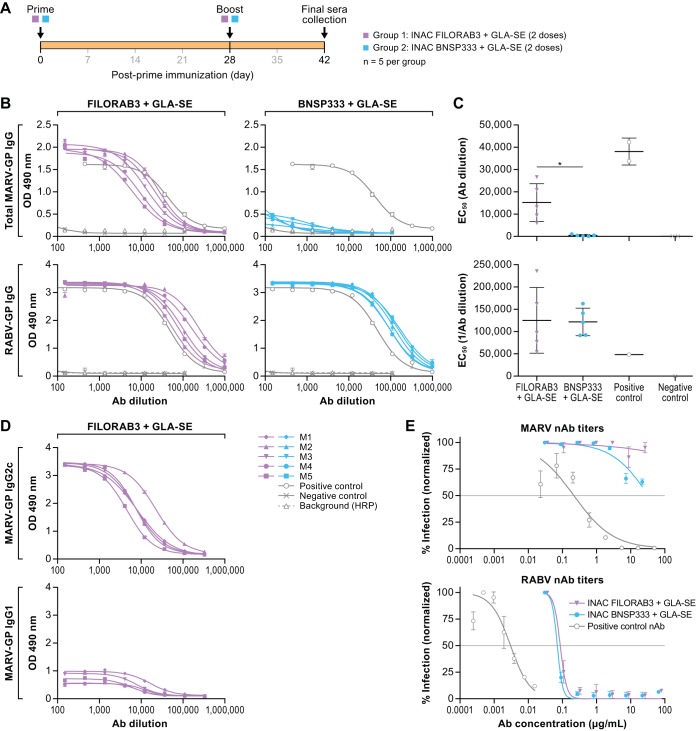
FIG 6.
Humoral response to FILORAB3. (A) Experimental timeline for immunization of C57BL/6 mice (n = 5 per group). C57BL/6 mice were immunized intramuscularly in the gastrocnemius muscle with a total of 10 μg each of either FILORAB3 or BNSP333 inactivated viral particles adjuvanted with 5 μg of a TLR-4 agonist in 2% stable emulsion (GLA-SE). All mice were primed on day 0 and boosted once on day 28. (B) Sera recovered from immunized mice at day 42 postinoculation were diluted 1:50 and analyzed in a 3-fold serial dilution via an indirect ELISA to test for the relative quantities of both MARV GP-specific (top) and RABV G-specific (bottom) antibodies. The OD490 values were compared to those for antigen-specific monoclonal antibody positive-control samples (gray open circle lines). (C) EC50 values from total MARV (top) and RABV (bottom) IgG ELISA curves were analyzed. Statistical significance was performed using an unpaired t test with Welch’s correction to compare the FILORAB3- and BNSP333-immunized groups. The results are presented as mean values. *, P < 0.05. (D) IgG2 and IgG1 isotype responses in FILORAB3- and BNSP333-immunized mice were assessed by ELISA at day 42 postinoculation to evaluate Th1- versus Th2-biased humoral immunity. (E) Purified IgG derived from pooled sera of FILORAB3- or BNSP333-immunized mice was analyzed in an in vitro pseudotyped lentivirus luciferase assay to determine the titers of MARV GP- and RABV G-neutralizing antibodies compared to those of a positive-control monoclonal antibody known to neutralize either MARV or RABV pseudotyped virus in vitro (gray lines). Graphs are representative of average data from three independent experiments. The gray horizontal lines indicate the threshold for a 50% reduction in infection. Ab, antibody; HRP, horseradish peroxidase.