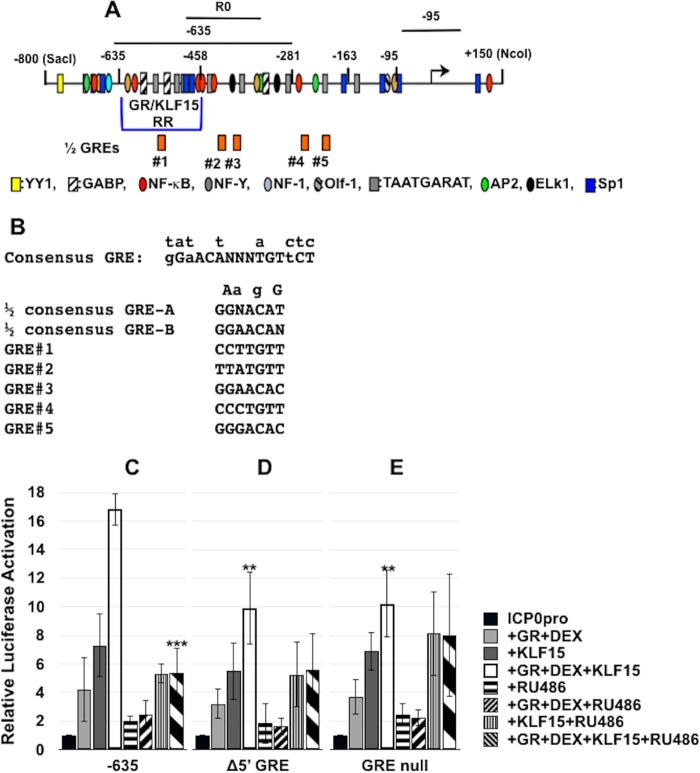
FIG 4.
Role of half-GREs in ICP0 promoter activation in transfected cells. (A) Schematic of ICP0 promoter that includes the location of the GR/KLF15 responsive region (GR/KLF15 RR), the locations of half-GREs, and sequences amplified by the respective PCR primers used in this study. Locations of transcription factor binding sites in the ICP0 promoter are indicated below the −635 ICP0 promoter construct. (B) DNA sequence of GRE consensus and partial GREs in the ICP0 promoter. Nucleotides above the consensus GRE indicate changes that still result in GR binding and transactivation, and “N” refers to any base. The putative half-GREs in the ICP0 promoter correspond to the numbered half-GREs in panel A. The Δ5′ GRE mutant has the TGT core sequence motif deleted. The GRE null mutant has deletions of all of the TGT or ACA core motifs present in the five half-GREs. (C to E) Neuro-2A cells were cultured in 2% stripped FBS after transfection with the −635 ICP0 promoter or half-GRE mutant promoters fused to the firefly luciferase gene. Shown is luciferase activity at 40 h after cells were cotransfected with the designated ICP0 promoter (0.5 μg of DNA), vector expressing a mouse GR (1.0 μg of DNA), and/or vector expressing human KLF15 (0.5 μg of DNA). Empty vector plasmid was added to certain samples to maintain the same amount of DNA in each transfection. Twenty-four hours following transfection, certain cultures were treated with DEX for 14 h (10 μM) and/or RU486 (10 μM). Dual-luciferase activity was determined as described in Materials and Methods. The results are the means from 3 independent experiments. Statistical analysis was performed as described in Materials and Methods. Unpaired t test was performed between the −635 ICP0 construct cotransfected with GR+DEX+KLF15 and indicated samples. **, P < 0.005; ***, P < 0.0005.