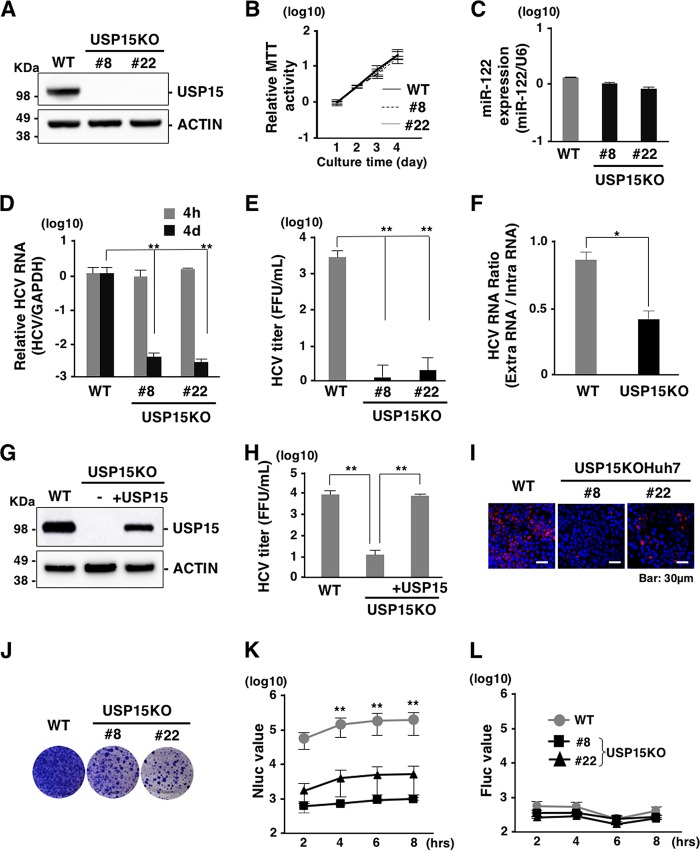
FIG 3.
Deficiency of USP15 impairs HCV propagation. (A) USP15-deficient Huh7 (USP15KOHuh7) cells were generated using a CRISPR/Cas9 system. The expression of USP15 was confirmed by immunoblotting. We successfully established two independent USP15KOHuh7 clones. (B) Wild-type (WT) and USP15KOHuh7 cells were seeded onto 24-well plates and incubated for 4 days. The cell growth of each cell line was analyzed by using an MTT [3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide] assay kit (Nacalai Tesque, Kyoto, Japan) according to the manufacturer’s protocol. (C) The expression of miR-122 in WT and USP15KOHuh7 cells was quantified by qRT-PCR. U6 RNA was used as the internal control. (D) WT and USP15KOHuh7 cells were infected with HCV at an MOI of 3. After 4 h or 4 days postinfection, the intracellular HCV RNA level was quantified by qRT-PCR using GAPDH mRNA as the internal control. (E) WT and USP15KOHuh7 cells were infected with HCV at an MOI of 3. After 4 days postinfection, infectious titers in the culture supernatants were determined by a focus-forming assay. (F) WT and USP15KOHuh7 cells were infected with HCV at an MOI of 3. After 4 days postinfection, extracellular and intracellular HCV RNA levels were quantified by qRT-PCR. The ratios between intracellular and extracellular HCV RNA levels were calculated as HCV RNA rates. (G) USP15KOHuh7 cells were transfected with a plasmid expressing FLAG-USP15, and USP15KOHuh7 cells expressing FLAG-USP15 were established. The expression of USP15 was confirmed by immunoblotting. (H) USP15KOHuh7 cells, cell lines with restored USP15, and Huh7 cells were infected with HCV. After 4 days postinfection, infectious titers in the culture supernatants were determined by a focus-forming assay. (I) HCV was inoculated into Huh7 cells or USP15KOHuh7 cells at an MOI of 3 and stained with anti-NS5A antibody at 4 days postinfection. (J) In vitro-transcribed HCV subgenomic replicon RNA (pSGR-JFH1) was electroporated into WT and USP15KOHuh7 cells, and the cells were incubated for 3 weeks in the presence of 1 mg/ml of G418. Colonies were visualized by Giemsa staining. (K) In vitro-transcribed HCV subgenomic replicon RNA (pSGR-NLuc-JFH1GND) was electroporated into WT and USP15KOHuh7 cells, and the activity of NLuc in culture supernatants was monitored. (L) In vitro-transcribed capped-FLuc RNA was electroporated into parental and USP15KOHuh7 cells, and the activity of FLuc in electroporated cells was monitored.