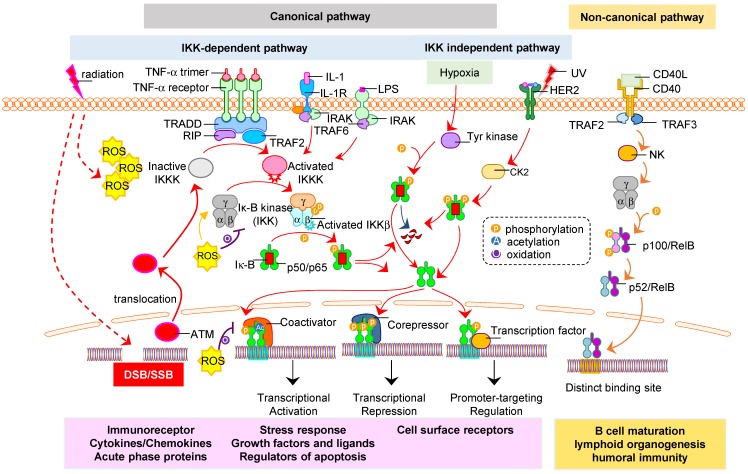
Figure 2.
Activation of NF-κB in ITME. Radiation can induce NF-κB activation in both tumor and stromal cells including immune cells in ITME. The subunits RelA (p65), c-Rel, RelB, p105/p50, and p100/p52 can form active homo- or hetero- dimeric complex and activated by the canonical and non-canonical pathway 62, 63. Radiation-induced NF-κB is mainly via canonical pathway that is divided into two categories: IKK-dependent or IKK-independent. The IκB is degraded upon phosphorylation by IKK which relocates NF-κB dimer (p50/p65) to nucleus for transcription of radiation-target genes including RAAPs such as CD47, HER2, and PD-1/PD-L1. UV or hypoxia activates NF-κB via IKK-independent phosphorylation of IκB in which RelB/p52 is activated via metabolism of p100. On the other hand, radiation-induced DNA damage activates nuclear ATM that also enhances NF-κB via regulation of IKK activity 8, 9, 64. The reactive oxygen species (ROS) produced by radiation triggers the NF-κB signaling in cytoplasm by IKK complex phosphorylation 65, while inhibits NF-κB signaling via oxidation of IKKɣ in cytoplasm or oxidation of p50 in nucleus. The canonical pathway impacts various biological processes, including immune response, inflammation, and cell growth and survival. The non-canonical pathway regulates important biological functions, including lymphoid organogenesis, B-cell maturation and DC activation.