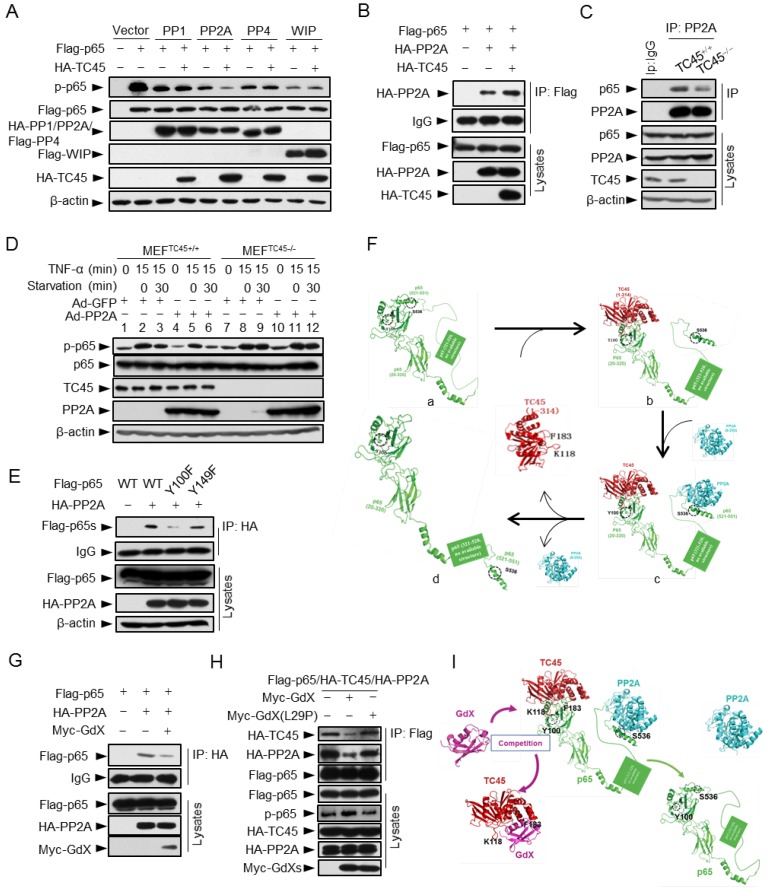
Figure 6.
TC45 mediates PP2A to dephosphorylation p-p65, which is interrupted by GdX. (A) TC45 and PP2A synergistically dephosphorylate p-p65. HEK293T cells were co-transfected with different phosphatases and Flag-tagged p65 plasmids. (B) Over-expression of TC45 enhanced the interaction of PP2A and p65. (C) The endogenous interaction of PP2A and p65 was decreased in TC45-deficient cells. (D) PP2A failed to dephosphorylate p-p65 without TC45. p-p65 levels were examined in MEFTC45+/+ and MEFTC45-/- cells infected with Ad-GdX or Ad-PP2A and treated with TNF-α (10 ng/mL) for 15 min, followed by TNF-α withdrawal for 30 min before harvesting. (E) Y100 is critical for the interaction of PP2A and p65. IP assays were performed after HEK-293T cells were transfected with the Flag-tagged p65, p65(Y100F), or p65(Y152F), as well as HA-PP2A for 24 h. (F) A molecular docking model showed TC45/p65/PP2A complex formation and disassociation. (a) The C-terminus of p65 is hided at the N-terminus of p65 to maintain an active form of p65. Without association of TC45 with p65 at its N-terminus, p65 remains a coated confirmation where the C-terminus, in particular S538, is hided. (b) TC45 starts to bind to the C-terminus of p65 and release the N-terminus of p65 from hiding. (c) PP2A gets a chance to associate with the released C-terminus and the heter-trimer complex of TC45/p65/PP2A is formed. In this complex, PP2A dephosphorylates S536 and finally maintains p65 unphosphorylated status (d). (G) GdX inhibited the interaction of p65 and PP2A. (H) GdX(L29P) mutant failed to decrease the interaction of PP2A and p65. HEK293T cells were transfected with the indicated plasmids for the IP experiment. (I) A model of the competition of GdX with TC45 to interact with p65. When TC45 interacts with the N-terminus of p65, the C-terminus of p65 is exposed to interact with PP2A. GdX interacts with TC45 and blocks its interaction with p65. In this way p65 maintains at its active form without interaction of PP2A.