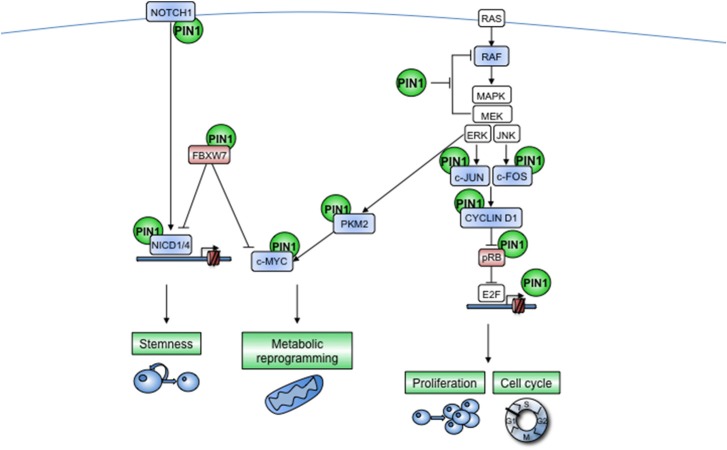
Figure 3.
PIN1 acts at multiple levels of cooperating cellular pathways. NOTCH receptor and RAS-dependent pathways are indicated as examples of signal transduction pathways that are regulated by PIN1 at multiple levels. Furthermore, PIN1 enables active cross-talk between different pathways, e.g., by promoting nuclear translocation of PKM2 after EGFR-stimulated phosphorylation by ERKs, that in turn serves to enhance c-MYC transcriptional activity. (Left) The NOTCH pathway is activated by a ligand-dependent cleavage of the membrane-bound receptor that releases the Notch intracellular domain (NICD), which translocates into the nucleus where it promotes the transactivation of several target genes. This signal is turned off by the E3-ubiquitin ligase FBXW7 as a safeguard mechanism against excessive NOTCH signaling. PIN1 is harnessed by the NOTCH pathway to sustain its own signaling, indeed PIN1 boosts the cleavage of the NOTCH1 receptor and prevents the interaction of NICD1 and NICD4 with FBXW7, increasing in this way pathway activation, protein stability and transcriptional activity. In some contexts, PIN1 can also directly block FBXW7 activity. Moreover, some NOTCH transcriptional targets, such as CYCLIN D1 and c-MYC, are also direct PIN1 targets, thus suggesting that PIN1 may amplify also the NOTCH transcriptional program. Notably NICD1 and NICD4 directly promote Pin1 transcription. Blue color indicates PIN1-activated proteins, red color indicates PIN1-inhibited proteins. Arrows and blocked lines show positive and negative effects, respectively. (Right) In response to growth stimuli, RAS activates the Raf kinase, which in turn activates downstream MAPKs that phosphorylate c-FOS and c-JUN causing the formation of the AP-1 transcription factor that transactivates the CYCLIN D1 gene. CYCLIN D1-CDK4/6 complex, in turn, blocks pRb, unleashing the transcription factor E2F, boosting in this way cell cycle progression. PIN1 itself is a direct transcriptional target of E2F and strongly promotes the RAS/MAPKs/CYCLIN/CDK cascade at multiple levels: (i) by blocking negative feed-back regulation of RAF by MAPKs, (ii) by enhancing both transcription and protein stability of CYCLIN D1, and (iii) by blocking the activity of pRB.