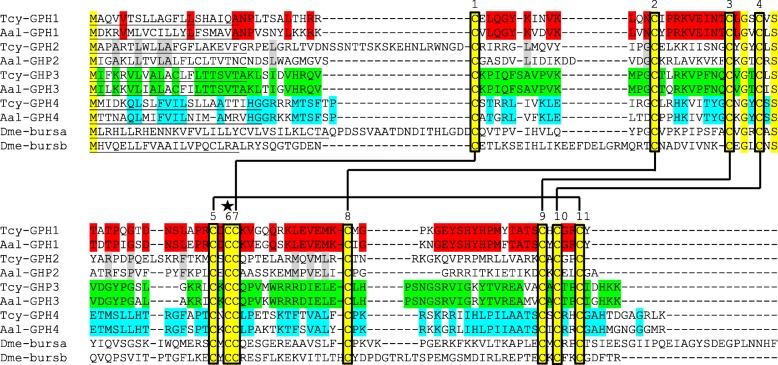
Fig. 7.
Alignment of the amino acid sequences from the glycoprotein hormones (GPHs) identified in the transcriptomes from T. cystophora and A. alatina, together with the Drosophila bursicon-α (Dme-bursα) and -β (Dme-bursβ) subunits. In T. cystophora, we discovered four glycoprotein hormone subunits (Tcy-GPH-1 to − 4) and the same number was found in A. alatina (Aal-GPH-1 to − 4). These subunits from one species can form hetero- or homodimers. Amino acid residues that were identical between two orthologues in the two cubomedusae are highlighted in the same color. Residues that are identical in all subunits are highlighted in yellow. GPHs are known to have five cystine bridges (presented as horizontal lines) formed by oxydation from ten cysteine residues (marked by vertical boxes). The star marks cysteine residue #6, which makes an intermolecular cystine bridge with the other subunit that is part of the dimer. It can be seen that the cystine bridges in the cubomedusan GPH and Drosophila bursicon subunits are probably the same. In addition, the cubomedusan subunits have several amino acid residues in common with the bursicon subunits, especially around cysteine residue #4. The sequences for Tcy-GPH-1 to − 4 have been submitted to the GenBank Data Bank with accession numbers MH835330-MH835333