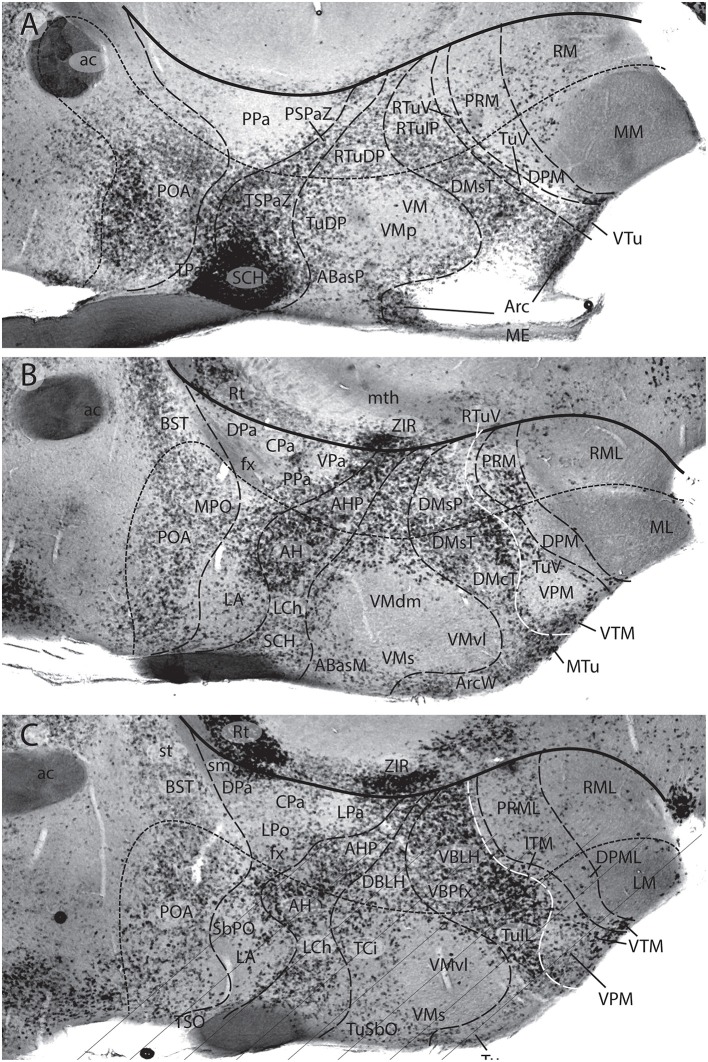
Figure 15.
Three sagittal sections adjacent to those in Figure 14, through the adult mouse hypothalamus, shown in medio-lateral sequence (A–C). They were in situ reacted for Gad67, a gene marker of GABAergic inhibitory neurons (from Puelles L. et al., 2012a; no copyright permission required). The thick black line indicates the hypothalamo-diencephalic boundary (same overall orientation as Figure 14). The nearly parallel line with minute dashes is the intrahypothalamic segmental boundary separating PHy from THy (or hp1 from hp2). Orthogonal dash lines separate alar and basal longitudinal progenitor domains. The overall image readily shows that the hypothalamic formations with marked numbers of excitatory neurons (Figure 14) have few if any GABAergic neurons (e.g., check LA, also most of the principal Pa nucleus, VM, VPM, DPM, MM, ML, LM; RM, RML—A–C). The maximal presence of GABAergic neurons appears at the SCH nucleus, and less markedly at the neighboring AH and AHP, all of them subparaventricular derivatives. The full dorsomedial formation across both THy and PHy is also rich in GABAergic neurons. The prethalamus (e.g., Rt; ZIR) also emerges as a GABAergic territory (B,C). There is also a shell of GABA cells around the VM nucleus (A–C). The preoptic area (POA) is also well provided with GABAergic neurons (A–C). Seeing the curved and topographically oblique (deformed) boundary lines that separate paraventricular from subparaventricular alar entities, as well as basal formations, one understands that the habitual atlas coronal sections are not helpful in understanding these alternative distributions highlighted by the selective molecular markers and more appropriate sagittal section planes.