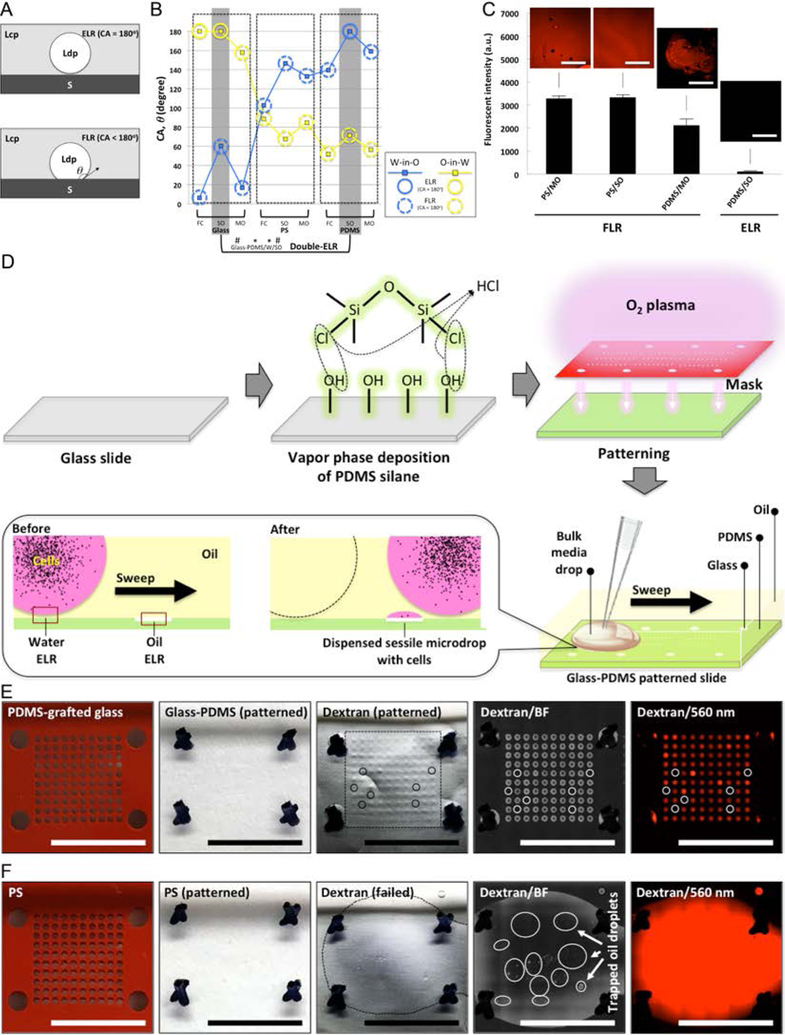
Fig. 1. ELR and FLR in multi-liquid-phase system and double-ELR-enabled underoil sweep patterning.
(A) Schematic of ELR (with CA, ϑ = 180°) and FLR (with ϑ < 180°). S - solid; Ldp - liquid of dispersed phase; Lcp - liquid of continuous phase. (B) CAs measured in water-in-oil (W-in-O, blue line) and oil-in-water (O-in-W, yellow line) conditions on three homogeneous substrates [glass (O2 plasma-treated), PS, and PDMS] with different oils (measurement error, ± 2°). Homogeneous substrate shows only single-ELR (e.g., glass/FC) at best to either water or oil. On heterogeneous substrate (by combining any two of the homogenous substrates, e.g., glass-PDMS/W/SO), double-ELR to both water and oil can be realized (table S2). (C) Fouling test on homogeneous substrates in ELR and FLR with four model systems (PS/MO, PS/SO and PDMS/MO for FLR; PDMS/SO for ELR). 1 mL dextran solution (Texas Red, 10000 MW, 1.0 mM in PBS) was added onto each substrate under oil and then removed shortly (< 10 seconds). Fluorescent intensity was recorded to check the residual of dye on substrate. It is clear only ELR effectively blocked substrate fouling or random sample loss from short contact with the dye solution. (D) Schematic of fabrication of glass-PDMS patterned slides and underoil sweep patterning based on double-ELR. The background (PDMS) shows ELR to water under oil. In contrast, the patterned area (glass) shows ELR to SO under water. A certain volume of culture media with cells (black dots) can be automatically and continuously dispersed onto spots by sweeping a bulk media across the substrate. Sweeping can be executed by dragging a liquid droplet across the surface with a pipet tip. (E) Underoil sweep patterning on a glass-PDMS patterned slide (containing 600 μm spots in a 10 × 10 array). 100 μL dextran solution was added to the spots via sweep then removed by a pipette. Unfilled (or empty) spots were labelled out with solid line circles. Variation of volume distribution measured in mean fluorescent intensity on each spot was shown in fig. S1. (F) Control with O2 plasma patterned PS. Sweep patterning became impractical due to FLR to water on PS under oil. 1 mL dextran solution was added instead to cover all of the spots then removed by a pipette. Discrete oil droplets trapped in the thin layer of aqueous solution were labelled out with solid line circles. In (E) and (F) from left to right: starting substrates (PDMS-grafted glass, PS) with silicone rubber mask (red), O2 plasma patterned substrates with mask removed, dextran-loaded substrates (the frames in black dashed line denote the solution sweeping or loading area on each substrate), media channel [brightfield (BF)] image, and dextran channel (560/607 nm) image. Scale bars: 500 μm in the insets of (C), 1 cm in (E) and (F).