Abstract
Background
Induction schedules in acute myeloid leukemia (AML) are based on combinations of cytarabine and anthracyclines. The choice of the anthracycline employed has been widely studied in multiple clinical trials showing similar complete remission rates.
Materials and Methods
Using an ex vivo test we have analyzed if a subset of AML patients may respond differently to cytarabine combined with idarubicin, daunorubicin or mitoxantrone. Bone marrow (BM) samples of 198 AML patients were incubated for 48 hours in 96 well plates, each well containing different drugs or drug combinations at different concentrations. Ex vivo drug sensitivity analysis was made using the PharmaFlow platform maintaining the BM microenvironment. Drug response was evaluated as depletion of AML blast cells in each well after incubation. Annexin V-FITC was used to quantify the ability of the drugs to induce apoptosis, and pharmacological responses were calculated using pharmacokinetic population models.
Results
Similar dose-respond graphs were generated for the three anthracyclines, with a slight decrease in EC50 with idarubicin (p=1.462E-06), whereas the interpatient variability of either drug was large. To identify those cases of selective sensitivity to anthracyclines, potency was compared, in terms of area under the curve. Differences in anthracycline monotherapy potency greater than 30% from 3 pairwise comparisons were identified in 28.3% of samples. Furthermore, different sensitivity was detected in 8.2% of patients comparing combinations of cytarabine and anthracyclines.
Discussion
A third of the patients could benefit from the use of this test in the first line induction therapy selection, although it should be confirmed in a clinical trial specifically designed.
Keywords: Anthracycline, ex-vivo test, Idarubicin, Daunorubicin, Mitoxantrone, Acute myeloid leukemia, Personalized medicine
Introduction
Induction 1st line schedules in de novo acute myeloid leukemia (AML) are based in a combination of an anthracycline with cytarabine (CYT) (3+7 schedule), obtaining complete remission (CR) rates of 70–80% after 1–2 cycles.1,2 Daunorubicin (DNR), idarubicin (IDA), mitoxantrone (MIT, an anthracenedione), and less frequently other anthracyclines have been employed in these schemes. The choice of the anthracycline employed has been widely studied in several randomized clinical trials (RCT),3–22 showing similar CR rates, with some exceptions in which IDA reported higher CR than DNR,4,6–8,12 finding reproduced in a Cochrane meta-analysis. 23
Different ex vivo tests have been employed to select the most effective drug combination from the individualized sensitivity and resistance assays, but none of them have been recommended in clinical practice.24 We are developing a Precision Medicine (PM) test based on an actionable native environment method (PharmaFlow platform), which showed excellent correlations with clinical responses in AML, avoiding some limitations of other ex vivo assays.25
The objective of this non-interventional study is to explore whether a significant percentage of patients AML samples may show different ex-vivo sensitivity to IDA vs DNR vs MIT combined with CYT.
Patients and Methods
Patients and study design
A multicenter, prospective, non-interventional cohort study was carried out in 33 Spanish institutions of the PETHEMA group. The inclusion period lasted five years (2012–2017), enrolling patients aged 18 years and older with newly diagnosed AML. Diagnosis and classification of AML were performed according to the World Health Classification (WHO) criteria.26 This study was approved by the Research Ethics Board of each participating institution and was conducted according to the Spanish law 14/2007 of biomedical research. Informed consent was provided to all patients.
Vivia’s PharmaFlow PM Test
• Native environment whole bone marrow sample
Ex vivo drug sensitivity analysis was made using the PharmaFlow platform (previously termed ExviTech®)25 maintaining the bone marrow (BM) microenvironment. A minimum BM sample volume between 1 and 2 ml was collected by aspiration at AML diagnosis, before starting induction chemotherapy, and was processed by an automated method in Vivia Biotech laboratories 24 hours after extraction. Samples were incubated for 48 hours in 96 well plates, each well containing different drugs or drug combinations at different concentrations, enabling calculation of dose-response curves for every single drug (CYT, IDA, DNR, MIT) and combination used in treatments (CYT-IDA, CYT-DNR, CYT-MIT). The number of BM samples analyzed were 289 with IDA, 333 with DNR and 274 with MIT. A more detailed description of the procedure has been published elsewhere.25 The concentrations assayed for each anthracycline were:
- Concentrations for IDA (μM): > 0.0002; 0.001; 0.002; 0.006; 0.01; 0.018; 0.02; 0.04; 0.05; 0.055; 0.08; 0.13; 0.16; 0.2; 0.26; 0.4; 0.5; 0.6; 1.5.
- Concentrations for DNR (μM): > 0.001; 0.05; 0.075; 0.093; 0.15; 0.18; 0.25; 0.3; 0.37; 0.45; 0.75; 0.85; 1.25; 1.5; 2.7; 3.
- Concentrations for MIT (μM): > 0.001; 0.0016; 0.008; 0.01; 0.04; 0.08; 0.2; 0.38; 0.6; 0.8; 1; 2.33; 3.5; 7.
• Modeling of ex vivo activity of CYT, IDA, DNR, MIT
Evaluation of drug response was done by counting the number of live pathological cells (LPC) remaining after incubation at increasing drug concentrations. Dying cells (apoptosis) were excluded using Annexin V-FITC. Pharmacological responses were estimated using pharmacodynamic (PD) population-based models27 which essentially perform the fitting of the dependent variable (natural log of LPC) in a non-linear mixed-effects model to derive typical population values (fixed effects) and the magnitude of inter-patient and residual variability (random effects). Model development was performed with the first-order conditional estimation method using interaction option with the software NONMEM (v7.2)28, according to the following equation:
Where LPC0 parameter refers to the number of LPC after incubation in the absence of drug, Emax represents the maximum fractional decrease in LPC that the drug can elicit, EC50, is the drug concentration exerting half of Emax, and γ is the parameter governing the steepness of the LPC vs drug concentration (C) curve. Potency (EC50) and efficacy (Emax) are PD parameters that characterize the pharmacological response and are integrated into a single value corresponding to the measurement of the area under the dose-response curve (Area Under the Curve, AUC).
For data presentation, the survival index was computed, with the number of LPC in control wells that were not exposed to any drugs being set as 100%. The number of live cells in each drug-treated well was compared with this control value, and the survival index for each drug at each concentration was determined as the percentage of LPC at every tested concentration.
Interpatient variability (IPV) associated with all parameters was described using an exponential model of the components of variance. An additive error structure was used for the residual variability. Population PD models were built with BM samples from 227 patients that were incubated with IDA, 271 with DNR, and 212 with MIT. Bayesian estimation methods were then used to retrieve individual patient parameters based on their available exposure-response measurements in conjunction with the PD population parameters. After several trials with different modeling strategies, we could conclude that optimal approach, in terms of correlation with clinical output, was achieved by forcing typical parameters to values obtained in a different model using a dataset from samples tested at 72h. Therefore, the typical parameter value for the maximum fractional effect (Emax) was set to 1 for both drugs. For γ, the typical parameter value was calculated but limited to the range 0–3. IPV for both parameters could not be determined with this dataset.
For interaction analysis, a Surface Interaction model29 was used to estimate the degree of synergy, referred as α parameter, between both drugs (R environment (v3.3.1) for statistical computing).30 In this analysis, a value equal to 0 is an additive effect, a value > 0 indicates a synergistic effect, and a value < 0 reflects an antagonistic effect.
Study endpoints
The primary end-point was the comparison between the selective sensitivities of the different anthracyclines individually using the AUCs in the dose-response curve. For the comparisons between the combinations of anthracyclines with CYT, we employed the volume under the surface (VUS) of the dose-response curves. Besides, the differences in either drug potency or synergism ex vivo were also calculated according to the observed and predicted response after induction.
Results
Patient Characteristics
Overall, 332 BM samples from patients with AML suspicion were received at the laboratory, from which 261 BM samples were completely monitored at the end of the study. Of them, 63 (24%) were not evaluable because of the following protocol issues: 1) incorrect informed consent form (32 patients), 2) no available case report form (23 patients), 3) misdiagnosis (3 patients), and 4) other unknown reasons (5 patients). Overall, clinical data from 198 patient’s samples (60%) were available at the end of this study. The main baseline characteristics of these patients are displayed in Table 1. In summary, the median age was 61 years (range, 19 to 91), all patients were newly diagnosed AML, and 37 patients (19%) were categorized as having high-risk cytogenetics. CR rate was obtained in 93 patients (47%), whereas 65 patients obtained partial remission or were resistant to induction.
Table 1.
Baseline characteristics of the 198 analyzed patients.
| Median | Range | |
|---|---|---|
| Age (years) | 61 | 19–91 |
| n | % | |
| 18–29 | 7 | 3.5 |
| 30–39 | 20 | 10.1 |
| 40–49 | 31 | 15.7 |
| 50–59 | 30 | 15.2 |
| >60 | 110 | 55.6 |
|
| ||
| Gender | n | % |
| Male | 113 | 57 |
| Female | 85 | 43 |
|
| ||
| ECOG | n | % |
| 0 | 57 | 29 |
| 1 | 73 | 37 |
| 2 | 24 | 12 |
| 3–4 | 8 | 4 |
| Unknown | 36 | 18 |
|
| ||
| FAB subtype | n | % |
| M0 | 14 | 7 |
| M1 | 46 | 23 |
| M2 | 44 | 22 |
| M4 | 32 | 16 |
| M5 | 27 | 14 |
| M6 | 2 | 1 |
| Unknown | 33 | 17 |
|
| ||
| Median | Range | |
| WBC (count × 109/L) | 18.65 | 0.6 – 270 |
| n | % | |
| 0–10 | 74 | 37 |
| 10–50 | 63 | 32 |
| >50 | 51 | 26 |
|
| ||
| Unknown | 10 | 5 |
|
| ||
| Cytogenetic risk profilea | n | % |
| Favorable | 15 | 8 |
| Intermediate | 111 | 56 |
| Adverse | 37 | 19 |
| Unknown | 35 | 18 |
|
| ||
| FLT3-ITD status | n | % |
| Wild | 119 | 60 |
| Mutant | 22 | 11 |
| Unknown | 57 | 29 |
|
| ||
| NPM1 status | n | % |
| Wild type | 92 | 46 |
| Mutant | 50 | 25 |
| Unknown | 56 | 28 |
|
| ||
| Response | n | % |
| CR/CRi | 93 | 47 |
| PR/resistance | 65 | 33 |
| Unknown | 40 | 20 |
Based on the risk groups described by Grimwade et al (2010).
ECOG-PS: Eastern Cooperative Oncology Group performance status; FAB: French-American-British classification; FLT3-ITD: fms-like tyrosine kinase 3-internal tandem duplication; NPM1: Nucleophosmin 1; WBC: white blood cells; PR: partial remission.
Ex vivo PharmaFlow Test characterization of IDA, DNR and MIT models
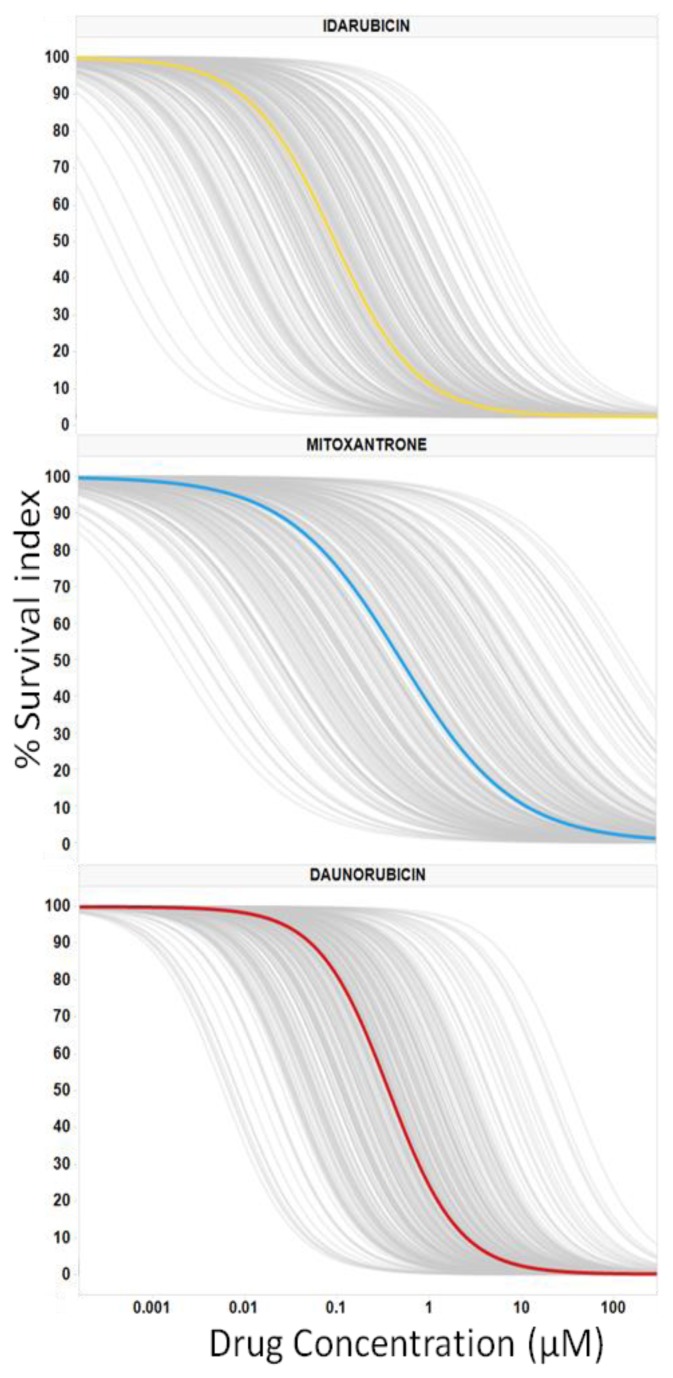
Dose-response graphs were generated for the single drugs (IDA, DNR, and MIT) using PD models (Figure 1). Most of the observations were contained within the simulation-based 95% confidence intervals of the 5–95th population percentiles proving good predictability of the selected models. Pharmacological population parameters, as well as variability and error values, are shown in Table 2.
Figure 1.
Average and Individual Dose Responses ex vivo for AML Drugs. Dose-Response Analysis was Completed for 3 Anthracyclines in Bone Marrow Samples From Patients With Acute Myeloid Leukemia; 227 with Idarubicin, 212 with Mitoxantrone and 271 with Daunorubicin. The Survival Index (y-Axis) Ranges From 100% to 0%, Displaying the Selective Acute Myeloid Leukemia Cell Depletion Calculated With Population Models. The Gray Lines Display Each Individual Response, With the Median Response Shown in yellow for Idarubicin, Panel (A); in blue for Mitoxantrone, Panel (B); and in red for Daunorubicin, Panel (C).
Table 2.
Estimates of the ex vivo population pharmacodynamic parameters. Parameters typical and random (variability and residual error percentage) are shown together with the corresponding relative standard error calculated as the ratio between the standard error provided by NONMEM and the estimate. Estimates of interpatient variability (IPV) are expressed as coefficient of variation (%).
| Parameter (units) | Mitoxantrone | Idarubicin | Daunorubicin |
|---|---|---|---|
| LPC0 (cells) | 7443 (10.04) | 8384 (14.18) | 7926 (10.21) |
| Emax (unitless) | 1 (−) | 1 (−) | 1 (−) |
| EC50 (μM) | 0.329* (16.84) | 0.07* (14.58) | 0.458*(12.08) |
| γ (unitless) | 0.77 (−) | 1.04 (−) | 1.13 (−) |
| Residual Error (log(μM)) | 845 (10.07) | 1027 (15.61) | 924 (11.79) |
|
| |||
| Inter-patient variability (IPV) | |||
|
| |||
| LPC0 | 86.4 (6.56) | 107.3 (6.83) | 92.9 (5.76) |
| Emax | N/D | N/D | N/D |
| EC50 | 224.2 (6) | 181.8 (5.46) | 168.6 (4.6) |
| γ | N/D | N/D | N/D |
| Residual Error | 83.1 (7.63) | 107.4 (7.21) | 97.4 (6.38) |
Emax: maximum fractional decrease in live pathological cells that the drug can elicit; EC50: drug concentration exerting half of Emax; LPC0: Starting live pathological cells in the absence of drug; N/D: not determined; γ: parameter governing the steepness of the LPC vs drug concentration curve.
p value = 1.462E-06
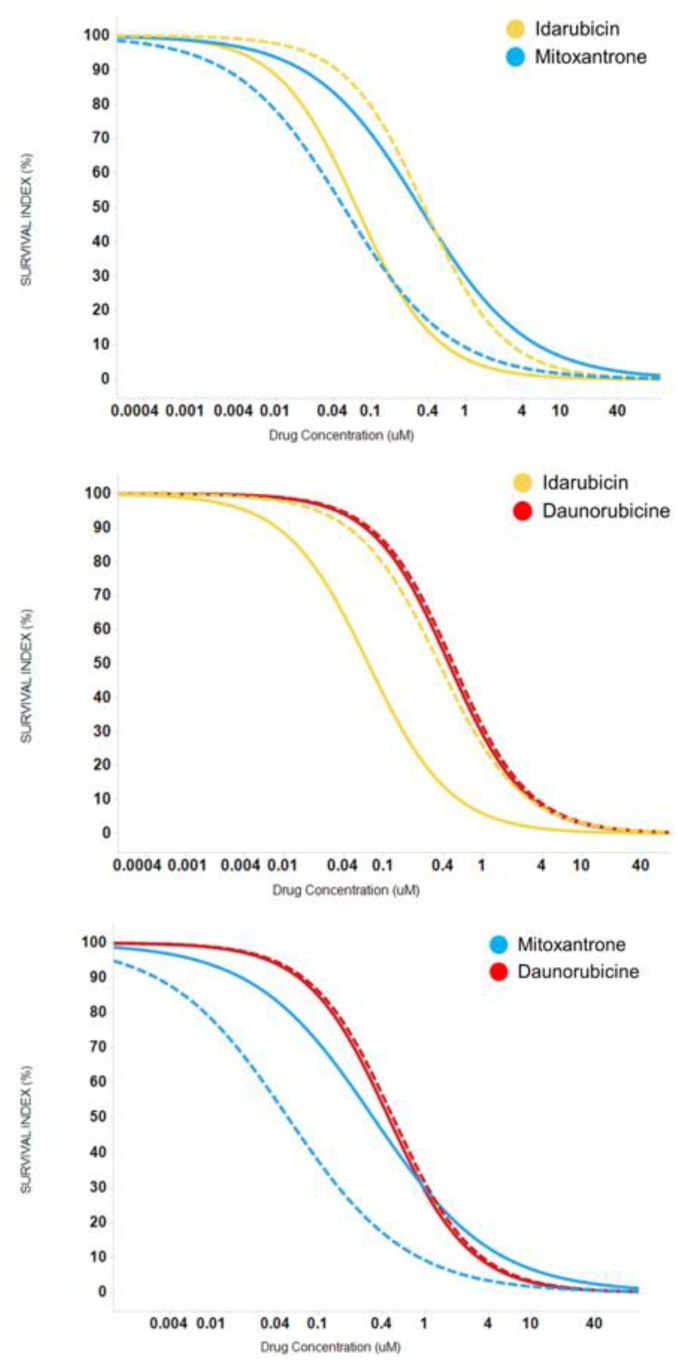
The average dose-responses of the three anthracyclines were similar, with a slight decrease in EC50 values with IDA (p-value = 1.462E-06; Table 2), reproducing the results of the clinical trials.4,6–8,12 However, the interpatient variability of either drug is quite large (Table 2, Figure 1), which could explain why some patients could show very differential sensitivities to these three drugs. As an example, Figure 2 illustrates a patient sample that is resistant to IDA and DNR (right shifted dose-response curve) but sensitive to MIT (left shifted dose-response curve).
Figure 2.
Example of differential individual sensitivities to anthracyclines. Dotted lines represented individual response to each drug and cotinuous lines the median response to each drug. Panel (A) shows an example of a patient resistant to Idarubicin (right shifted dose response curve) but sensitive to Mitoxantrone (left shifted dose response curve). Panel (B) shows an example of a patient resistant to Idarubicin and Daunorubicin (right shifted dose response curve). Panel (C) shows an example of a patient resistant to Daunorubicin (right shifted dose response curve) but sensitive to Mitoxantrone (left shifted dose response curve).
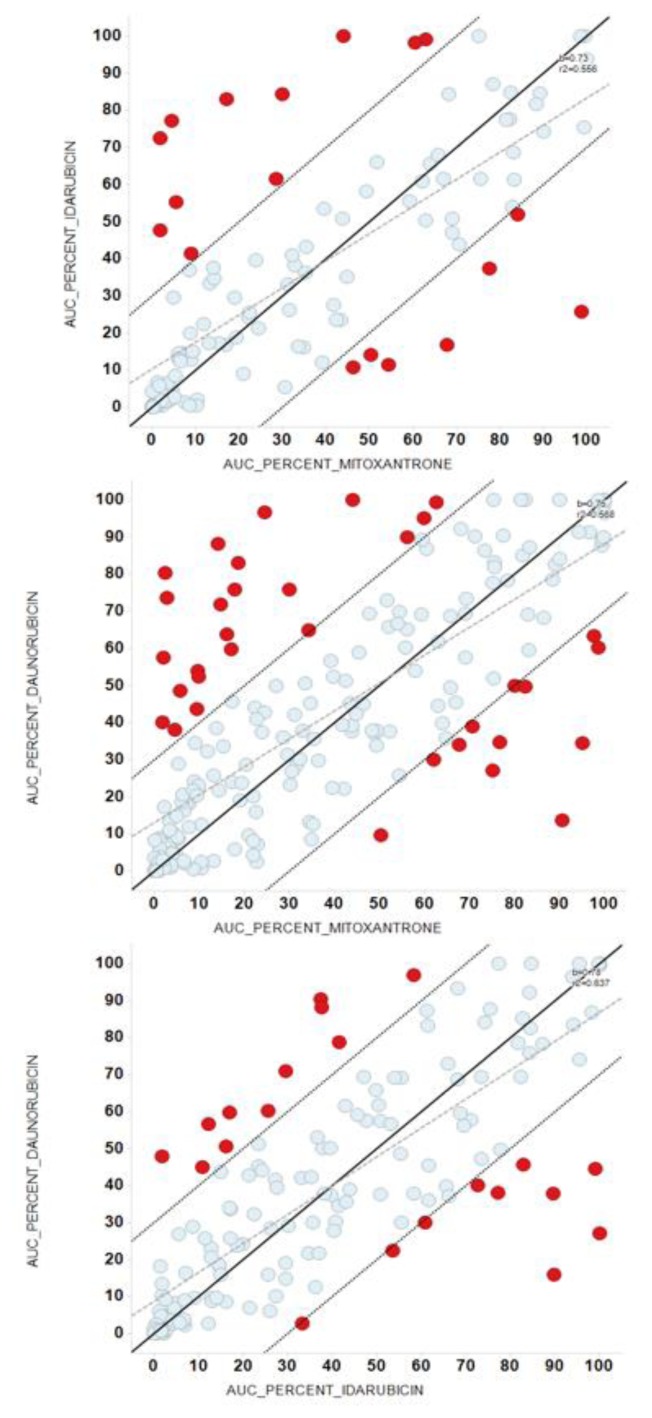
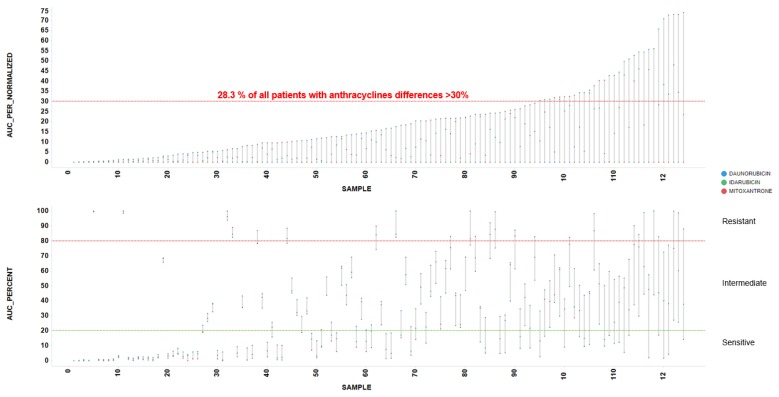
To identify these cases of selective sensitivity to anthracyclines, we compared the potency, regarding AUC, between IDA vs. DNR, IDA vs. MIT, and DNR vs. MIT (Figure 3, Table 3). Most dots tend to line up, but red dots represent patient samples with a difference in potency between these drugs >30%. Red dots from 3 pairwise comparisons identify 28.3% of patient samples with >30% different potency among IDA-DNR-MIT (Figure 4).
Figure 3.
Comparison of the potency between anthracyclines. Panels A-C represented the pairwise comparisons between Area Under (AUC) the Dose-Response Curve of the anthracyclines, with their bisectors, linear regression lines and R2 values. Red dots represent patient samples with a difference in potency between these drugs greater than 30%. Panel (A) comparison between AUCs of Idarubicin and Mitoxantrone; Panel (B) comparison between AUCs of Daunorubicin and Mitoxantrone; Panel (C) comparison between AUCs of Daunorubicin and Idarubicin.
Table 3.
Differences in Area Under the Dose-Response Curve between anthracyclines.
| AUC | ||||
|---|---|---|---|---|
|
| ||||
| Over 30% | Normal | Total | % | |
| DNR_IDA | 15 | 102 | 117 | 12.82 |
| DNR_MIT | 32 | 172 | 204 | 15.69 |
| IDA_MIT | 17 | 100 | 117 | 14.53 |
AUC: area under the curve; DNR: daunorubicin; IDA: idarubicin; MIT: mitoxantrone.
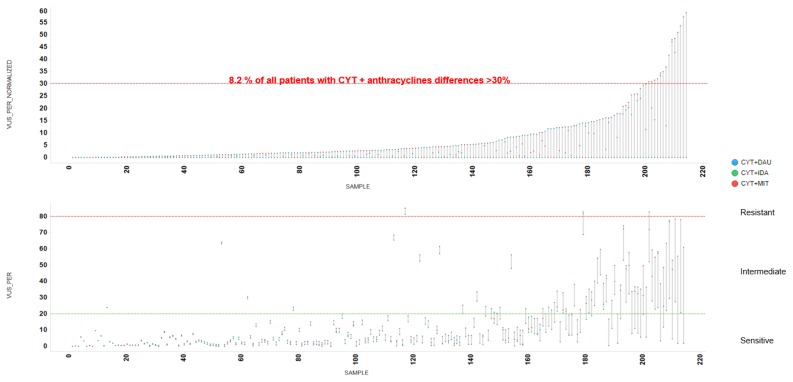
Figure 4.
Differences in Area Under the Dose-Response Curve between anthracyclines. A 28.3% of patients samples showed >30% different potency among Idarubicin-Daunorubicin-Mitoxantrone Area Under the Dose-Response Curve (AUC).
Ex vivo PharmaFlow Test characterization of CYT-IDA, CYT-DNR, and CYT-MIT combinations and their synergism
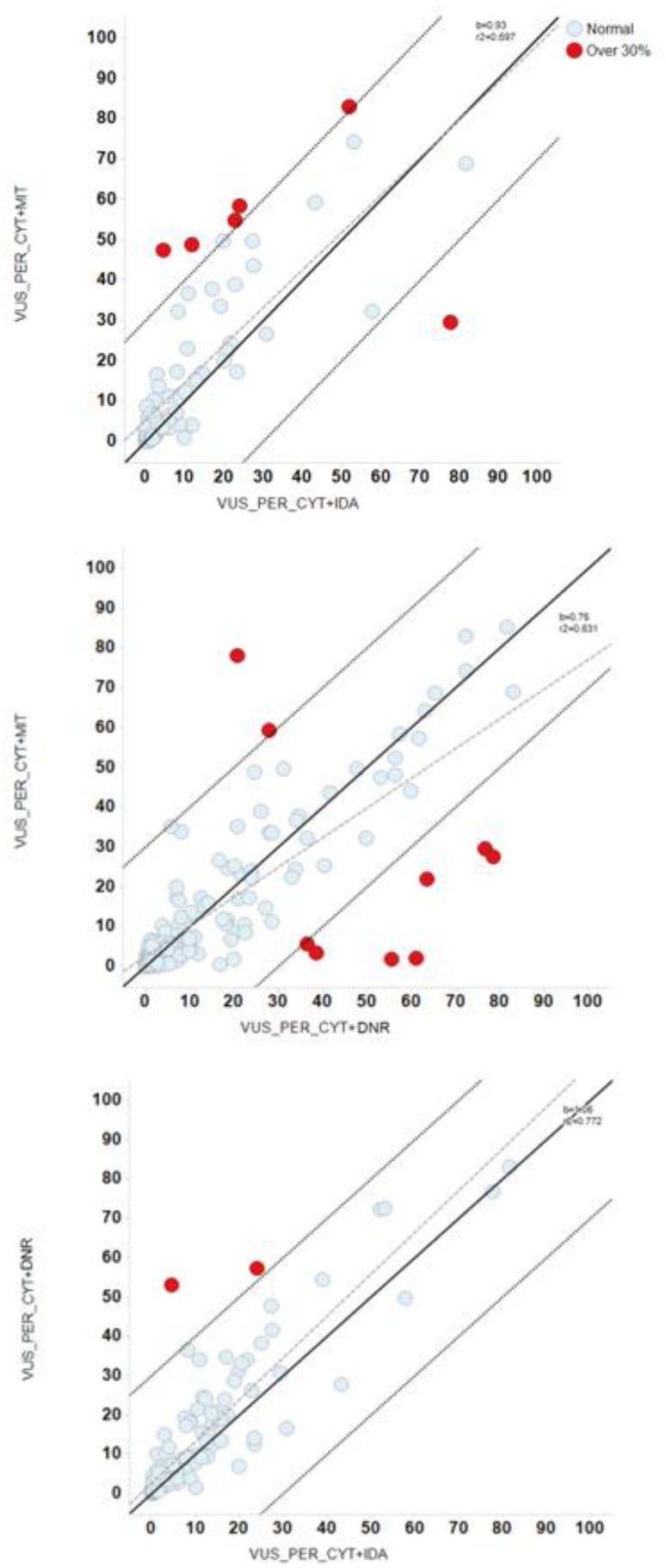
The pairwise comparison of the combination treatments CYT-IDA, CYT-DNR, and CYT-MIT obtained differential sensitivity to these anthracyclines (red dots of Figure 5). In this case, the red dots represent patient samples with a difference in CYT + anthracyclines synergy differences >30%, and red dots from 3 pairwise comparisons identified an 8.2% of patient samples (Figure 6, Table 4).
Figure 5.
Comparison of the potency between combinations of cytarabine and anthracyclines. Panels A–C represented the pairwise comparisons between Volume Under the Surface (VUS) of the combinations of cytarabine (CYT) with anthracyclines, with their bisectors, linear regression lines and R2 values. Red dots represent patient samples with a difference in potency between these drugs greater than 30%. Panel (A) comparison between VUS of Cytarabine + Mitoxantrone (CYT+MIT) and Cytarabine + Idarubicin (CYT+IDA); Panel (B) comparison between VUS of CYT+MIT and Cytarabine + Daunorubicin (CYT+DNR); Panel (C) comparison between VUS of CYT+DNR and CYT+IDA.
Figure 6.
Differences in Volume Under the Surface between combinations of cytarabine and different anthracyclines. An 8.2 % of patients samples obtained >30% of different sensitivity in Volume Under the Surface (VUS) of Cytarabine + Idarubicin (CYT+IDA), Cytarabine + Daunorubicin (CYT+DNR) and Cytarabine + Mitoxantrone (CYT+MIT).
Table 4.
Differences in Volume Under the Surface (VUS) between the combinations of cytarabine and different anthracyclines.
| VUS | ||||
|---|---|---|---|---|
|
| ||||
| Over 30% | Normal | Total | % | |
| CYT+DNR_CYT+IDA | 2 | 125 | 127 | 1.57 |
| CYT+MIT_CYT+IDA | 6 | 81 | 87 | 6.90 |
| CYT+DNR_CYT+MIT | 9 | 153 | 162 | 5.56 |
CYT: cytarabine; DNR: daunorubicin; IDA: idarubicin; MIT: mitoxantrone; VUS: volume under the surface.
Furthermore, the values for the alpha parameters of the interaction models of CYT-IDA, CYT-MIT, CYT-DNR were 0.72, 0.59 and 0.25, indicating synergistic response in the ex vivo combination experiments.
Discussion
The findings of this study show that PharmaFlow PM test seems able to identify a subset of AML patients who have a significantly different ex vivo pharmacological response to anthracycline drugs. We can hypothesize that if these selective anthracycline ex vivo responses were translated to in vivo responses, a fraction of this 28.3% subpopulation could benefit significantly from receiving a specific anthracycline-based on the ex vivo test sensitivity results. Furthermore, an 8.2% of patients showed a significant difference in the synergy between CYT and anthracyclines, in which the choice of the anthracycline could be crucial.
The first line induction therapy recommended by ELN1 and NCCN2 clinical guidelines includes seven days of a standard dose of CYT plus three days of an anthracycline, especially IDA (12 mg/m2) or DNR (60–90 mg/m2). The combination of CYT-MIT was not considered standard therapy, although it has been widely employed.
The influence of the anthracycline’s selection in the efficacy of induction therapy was analyzed in some RCTs.3–22 The comparison between CYT-DNR and CYT-IDA has been studied in 13 different trials,3–15 but only five studies reported differences in CR rates in favor of CYT-IDA.4,6–8,12 A meta-analysis confirmed the superiority of CYT-IDA against CYT-DNR, obtaining higher overall survival (OS), disease-free survival (DFS), CR, lower relapse rate, although this scheme increased induction death and mucositis.23 Regarding the employment of CYT-DNR or CYT-MIT, a clinical trial reported similar CR, length of duration of CR, OS, and toxicity.16 No evidence of differences between CYT-IDA and CYT-MIT in CR, survival rates, and toxicity was observed in 6 RCTs9,11,17–20 and one meta-analysis.23 Combinations of CYT-doxorubicin showed worse outcomes than CYT-DNR21 and CYT-IDA.22 According to clinical trials, in our study the average dose-responses of IDA, DNR, and MIT were similar, with a slight decrease in EC50 with IDA, indicating a probable higher potency with IDA than DNR and MIT. However, the anthracycline dosage of induction protocols assumed a cumulative doses proportion of 4:1 for DNR: IDA and DNR: MIT,31 but these proportions are not based in well-designed trials. In our cohort, according to this proportion and EC50 of DNR (0.458), the estimated EC50 of IDA and MIT was 0.115, a proportion 1.6 fold higher than IDA EC50 and three fold lower than MIT EC50 measured with ex vivo test.
Other studies analyzed the role of different anthracyclines in the AML induction with CYT and a third component, but CR and survival rates were similar for DNR, MIT, and aclarubicin.32,33 Besides the selection of the anthracycline, the dose intensity is crucial in the therapy success. An RCT34 reported significant improvements in CR, OS and event-free survival (EFS) using DNR doses of 90 mg/m2 compared to doses of 45 mg/m2. The response-oriented individualized induction therapy is another approach tested with IDA+CYT scheme without any advantage over the standard scheme.35 In addition, some specific AML characteristics could modify the anthracycline response, such as FLT3-ITD mutated patients which showed higher CR and survival with high-dose DNR compared to standard-dose DNR or IDA.36,37 These findings were reproduced in vitro in FLT3-ITD-mutated cell lines.37 Unfortunately, we have not enough data to analyze the impact of this mutation in our cohort.
Despite the previous experiences of ex vivo drug testing with limited sensitivity38–44, the PharmaFlow PM test aims to solve technical limitations including some novelties25:
the use of whole BM sample, maintaining the native environment, which has been hypothesized that it can influence the emergence of resistance;45–48
the increase of the accuracy obtained modeling ex vivo activity with PD population models in one single step;49
the improvements in the measures performed by automated flow cytometry platform (PharmaFlow).
The correlation between in vitro and in vivo therapy sensitivity of PharmaFlow PM test has been recently demonstrated in a cohort of 123 AML patients after induction therapy with CYT-IDA (most of these patients were also included in this study).50 This study achieved an 81% of overall accuracy in the correlations between test predictions and hematological response, identifying with success responders (CR/CR with incomplete recovery) in 93% of cases and non-responders (partial remission/resistance) in 60% of cases. The present study generates a theoretical role of PM tests in individual anthracycline selection but does not provide enough data and critical analyses to allow to translate their use in the routine clinical practice.
Regarding the synergism between anthracyclines and CYT, we observed a synergistic response with the three combinations, especially with CYT-IDA and CYT-MIT. In a previous study, we also reported a higher synergy with CYT-IDA and CYT-MIT combination and a trend to an additive effect with CYT-DAU.25 Curiously, a novel approach in AML therapy is the use of the liposomal formulation of CYT and DNR in a molar ratio concentration of 5:1, based on a probable higher synergistic effect.51,52 Furthermore, the pairwise comparisons between combinations of CYT-IDA, CYT-DNR, and CYT-MIT found in an 8.2% of patients synergy differences >30%, probably associated to the interpatient variability in drug sensibility observed in dose-response graphs.
Some limitations should be addressed in this study. First, this study analyzes the differences between ex vivo sensitivities to three different anthracyclines combined with CYT in BM samples of AML patients at diagnosis, but the correlation between ex vivo responses and clinical response was not analyzed. Second, although the incubation time was relatively short, additional transportation and processing time could lead, in several patients, to a non-affordable delay to start induction chemotherapy while receiving the test report. Third, associations of the different in vitro response of each anthracycline and specific characteristics of AML (age, WBC, cytogenetic risk, FLT3-ITD, and NPM1 status, etc.) were not analyzed. Finally, the findings reported are not yet validated in an independent cohort.
Conclusions
The ex vivo PharmaFlow PM test obtained in a 28.3% of the BM samples analyzed overall differences in sensitivity to anthracyclines in monotherapy. This test could allow designing a trial to explore a personalized selection of anthracycline therapy in AML patients. A similar approach is being tested in a clinical trial by PETHEMA group in relapsed or refractory AML patients to select the salvage therapy based on the ex vivo sensitivity to conventional chemotherapy agents. The role an adequate selection in this subset of AML patients is critical because none of the salvage regimens53 has achieved outstanding CR rates, long-lasting remissions, and acceptable OS.
Acknowledgments
We are grateful to all participating institutions and clinicians in the PETHEMA group, and all the patients included.
Footnotes
Competing interests: The authors have declared that no competing interests exist.
References
- 1.Döhner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Büchner T, Dombret H, Ebert BL, Fenaux P, Larson RA, Levine RL, Lo-Coco F, Naoe T, Niederwieser D, Ossenkoppele GJ, Sanz M, Sierra J, Tallman MS, Tien HF, Wei AH, Löwenberg B, Bloomfield C. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424–7. doi: 10.1182/blood-2016-08-733196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Donnell MR, Tallman MS, Abboud CN, Altman JK, Appelbaum FR, Arber DA, Bhatt V, Bixby D, Blum W, Coutre SE, De Lima M, Fathi AT, Fiorella M, Foran JM, Gore SD, Hall AC, Kropf P, Lancet J, Maness LJ, Marcucci G, Martin MG, Moore JO, Olin R, Peker D, Pollyea DA, Pratz K, Ravandi F, Shami PJ, Stone RM, Strickland SA, Wang ES, Wieduwilt M, Gregory K, Ogba N. Acute Myeloid Leukemia, Version 3.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2017;15:926–57. doi: 10.6004/jnccn.2017.0116. [DOI] [PubMed] [Google Scholar]
- 3.Petti MC, Mandelli F. Idarubicin in acute leukemias: experience of the Italian Cooperative Group GIMEMA. Semin Oncol. 1989;16:10–5. [PubMed] [Google Scholar]
- 4.Berman E, Heller G, Santorsa J, McKenzie S, Gee T, Kempin S, Gulati S, Andreeff M, Kolitz J, Gabrilove J, et al. Results of a randomized trial comparing idarubicin and cytosine arabinoside with daunorubicin and cytosine arabinoside in adult patients with newly diagnosed acute myelogenous leukemia. Blood. 1991;77:1666–74. [PubMed] [Google Scholar]
- 5.Mandelli F, Petti MC, Ardia A, Di Pietro N, Di Raimondo F, Ganzina F, Falconi E, Geraci E, Ladogana S, Latagliata R, et al. A randomised clinical trial comparing idarubicin and cytarabine to daunorubicin and cytarabine in the treatment of acute non-lymphoid leukaemia. A multicentric study from the Italian Co-operative Group GIMEMA. Eur J Cancer. 1991;27:750–5. doi: 10.1016/0277-5379(91)90181-C. [DOI] [PubMed] [Google Scholar]
- 6.Vogler WR, Velez-Garcia E, Weiner RS, Flaum MA, Bartolucci AA, Omura GA, Gerber MC, Banks PL. A phase III trial comparing idarubicin and daunorubicin in combination with cytarabine in acute myelogenous leukemia: A Southeastern Cancer Study Group study. J Clin Oncol. 1992;10:1103–11. doi: 10.1200/JCO.1992.10.7.1103. [DOI] [PubMed] [Google Scholar]
- 7.Wiernik PH, Banks PLC, Case DC, Jr, Arlin ZA, Periman PO, Todd MB, Ritch PS, Enck RE, Weitberg AB. Cytarabine plus idarubicin or daunorubicin as induction and consolidation therapy for previously untreated adult patients with acute myeloid leukemia. Blood. 1992;79:313–9. [PubMed] [Google Scholar]
- 8.Reiffers J, Huguet F, Stoppa AM, Molina L, Marit G, Attal M, Gastaut JA, Michallet M, Lepeu G, Broustet A, Pris J, Maraninchi D, Hollard D, Fabères C, Mercier M, Hurteloup P, Danel P, Tellier Z, Berthaud P. A prospective randomized trial of idarubicin vs daunorubicin in combination chemotherapy for acute myelogenous leukemia of the age group 55 to 75. Leukemia. 1996;10(3):389–95. [PubMed] [Google Scholar]
- 9.Rowe JM, Neuberg D, Friedenberg W, Bennett JM, Paietta E, Makary AZ, Liesveld JL, Abboud CN, Dewald G, Hayes FA, Tallman MS, Wiernik PH Eastern Cooperative Oncology. A phase 3 study of three induction regimens and of priming with GM-CSF in older adults with acute myeloid leukemia: A trial by the Eastern Cooperative Oncology Group. Blood. 2004;103:479–85. doi: 10.1182/blood-2003-05-1686. [DOI] [PubMed] [Google Scholar]
- 10.Gardin C, Turlure P, Fagot T, Thomas X, Terre C, Contentin N, Raffoux E, de Botton S, Pautas C, Reman O, Bourhis JH, Fenaux P, Castaigne S, Michallet M, Preudhomme C, de Revel T, Bordessoule D, Dombret H. Postremission treatment of elderly patients with acute myeloid leukemia in first complete remission after intensive induction chemotherapy: Results of the multicenter randomized Acute Leukemia French Association (ALFA) 9803 trial. Blood. 2007;109:5129–35. doi: 10.1182/blood-2007-02-069666. [DOI] [PubMed] [Google Scholar]
- 11.Mandelli F, Vignetti M, Suciu S, Stasi R, Petti MC, Meloni G, Muus P, Marmont F, Marie JP, Labar B, Thomas X, Di Raimondo F, Willemze R, Liso V, Ferrara F, Baila L, Fazi P, Zittoun R, Amadori S, de Witte T. Daunorubicin versus mitoxantrone versus idarubicin as induction and consolidation chemotherapy for adults with acute myeloid leukemia: The EORTC and GIMEMA groups study AML-10. J Clin Oncol. 2009;27:5397–403. doi: 10.1200/JCO.2008.20.6490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pautas C, Merabet F, Thomas X, Raffoux E, Gardin C, Corm S, Bourhis JH, Reman O, Turlure P, Contentin N, de Revel T, Rousselot P, Preudhomme C, Bordessoule D, Fenaux P, Terré C, Michallet M, Dombret H, Chevret S, Castaigne S. Randomized study of intensified anthracycline doses for induction and recombinant interleukin-2 for maintenance in patients with acute myeloid leukemia age 50 to 70 years: Results of the ALFA- 9801 study. J Clin Oncol. 2010;28:808–14. doi: 10.1200/JCO.2009.23.2652. [DOI] [PubMed] [Google Scholar]
- 13.Ohtake S, Miyawaki S, Fujita H, Kiyoi H, Shinagawa K, Usui N, Okumura H, Miyamura K, Nakaseko C, Miyazaki Y, Fujieda A, Nagai T, Yamane T, Taniwaki M, Takahashi M, Yagasaki F, Kimura Y, Asou N, Sakamaki H, Handa H, Honda S, Ohnishi K, Naoe T, Ohno R. Randomized study of induction therapy comparing standard-dose idarubicin with high-dose daunorubicin in adult patients with previously untreated acute myeloid leukemia: The JALSGAML201 study. Blood. 2011;117:2358–65. doi: 10.1182/blood-2010-03-273243. [DOI] [PubMed] [Google Scholar]
- 14.Creutzig U, Zimmermann M, Bourquin J-P, Dworzak MN, Fleischhack G, Graf N, Klingebiel T, Kremens B, Lehrnbecher T, von Neuhoff C, Ritter J, Sander A, Schrauder A, von Stackelberg A, Starý J, Reinhardt D. Randomized trial comparing liposomal daunorubicin with idarubicin as induction for pediatric acute myeloid leukemia: results from Study AML-BFM 2004. Blood. 2013;122:37–43. doi: 10.1182/blood-2013-02-484097. [DOI] [PubMed] [Google Scholar]
- 15.Récher C, Béné MC, Lioure B, Pigneux A, Vey N, Delaunay J, Luquet I, Hunault M, Guyotat D, Bouscary D, Fegueux N, Jourdan E, Lissandre S, Escoffre-Barbe M, Bonmati C, Randriamalala E, Guièze R, Ojeda-Uribe M, Dreyfus F, Harousseau JL, Cahn JY, Ifrah N, Guardiola P Groupe Ouest-Est d’ étude des Leucé mies Aiguës et autres. Long-term results of a randomized phase 3 trial comparing idarubicin and daunorubicin in younger patients with acute myeloid leukaemia. Leukemia. 2014;28:440–3. doi: 10.1038/leu.2013.290. [DOI] [PubMed] [Google Scholar]
- 16.Pavlovsky S, Gonzalez Llaven J, Sobrevilla P, Eppinger-Helft M, Marin A, López-Hernández M, Fernandez I, Rubio ME, Ibarra S, et al. A randomized study of mitoxantrone plus cytarabine versus daunomycin plus cytarabine in the treatment of previously untreated adult patients with acute nonlymphocytic leukemia. Ann Hematol. 1994;69:11–5. doi: 10.1007/BF01757342. [DOI] [PubMed] [Google Scholar]
- 17.Beksac M, Arslan O, Koc H, Akan H, Ilhan O, Arat M, Ozcan M, Gürman G, Konuk N, Uysal A. Randomised unicenter trial for comparison of three regimens in de novo adult acute nonlymphoblastic leukaemia. Med Oncol. 1998;15:183–90. doi: 10.1007/BF02821937. [DOI] [PubMed] [Google Scholar]
- 18.Archimbaud E, Jehn U, Thomas X, De Cataldo F, Fillet G, Belhabri A, Peaud PY, Martin C, Amadori S, Willemze R. Multicenter randomized phase II trial of idarubicin vs mitoxantrone, combined with VP-16 and cytarabine for induction/consolidation therapy, followed by a feasibility study of autologous peripheral blood stem cell transplantation in elderly patients with acute myeloid leukemia. Leukemia. 1999;13:843–9. doi: 10.1038/sj.leu.2401445. [DOI] [PubMed] [Google Scholar]
- 19.Indrak K, Hubacek J, Mayer J, Voglová J, Jarosová M, Krahulová M, Malý J, Faber E, Penka M, Kmonícek M, Jebavý L, Szotkowski T, Knotková R, Hlusí A, Zapletalová J. Comparison of the effectiveness of idarubicin (Zavedos) and mitoxantrone (Refador) in induction therapy of acute myeloid leukemia in elderly patients (55–75) (a prospective multicenter randomized study conducted 1998–2000. Vnitr Lek. 2001;47:48–56. [PubMed] [Google Scholar]
- 20.De Moerloose B, Suciu S, MunzerPiette C, Yakouben K, Margueritte G, Lutz P, Uyttebroeck A, Rohrlich P, Ferster A, Boutard P, Dresse MF, Rialland X, Norton L, Sirvent N, Karrasch M, Benoit Y, Bertrand Y. Similar efficacy and toxicity profile for idarubicin and mitoxantrone in induction and intensification treatment of children with acute myeloid leukemia (AML) or myelodysplasia (MDS): Long-term results of the EORTC-CLG randomized phase III trial 58921. Blood. 2011;118 Abstract 2615. [Google Scholar]
- 21.Yates J, Glidewell O, Wiernik P, Cooper MR, Steinberg D, Dosik H, Levy R, Hoagland C, Henry P, Gottlieb A, Cornell C, Berenberg J, Hutchison JL, Raich P, Nissen N, Ellison RR, Frelick R, James GW, Falkson G, Silver RT, Haurani F, Green M, Henderson E, Leone L, Holland JF. Cytosine arabinoside with daunorubicin or adriamycin for therapy of acute myelocytic leukemia: a CALGB study. Blood. 1982;60:454–62. [PubMed] [Google Scholar]
- 22.Bezwoda WR, Dansey RD. Idarubicin plus cytarabine versus doxorubicin plus cytarabine in induction therapy for acute non-lymphoid leukaemia: A randomized trial. Leuk Lymphoma. 1990;1:221–5. doi: 10.3109/10428199009042483. [DOI] [PubMed] [Google Scholar]
- 23.Li X, Xu S, Tan Y, Chen J. The effects of idarubicin versus other anthracyclines for induction therapy of patients with newly diagnosed leukaemia. Cochrane Database Syst Rev. 2015;(6):CD010432. doi: 10.1002/14651858.CD010432.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schrag D, Garewal HS, Burstein HJ, Samson DJ, Von Hoff DD, Somerfield MR ASCO Working Group on Chemotherapy Sensitivity and Resistance Assays. American Society of Clinical Oncology Technology Assessment: chemotherapy sensitivity and resistance assays. J Clin Oncol. 2004;22:3631–8. doi: 10.1200/JCO.2004.05.065. [DOI] [PubMed] [Google Scholar]
- 25.Bennett TA, Montesinos P, Moscardo F, Martinez-Cuadron D, Martinez J, Sierra J, García R, de Oteyza JP, Fernandez P, Serrano J, Fernandez A, Herrera P, Gonzalez A, Bethancourt C, Rodriguez-Macias G, Alonso A, Vera JA, Navas B, Lavilla E, Lopez JA, Jimenez S, Simiele A, Vidriales B, Gonzalez BJ, Burgaleta C, Hernandez Rivas JA, Mascu-ano RC, Bautista G, Perez Simon JA, Fuente Ade L, Rayón C, Troconiz IF, Janda A, Bosanquet AG, Hernandez-Campo P, Primo D, Lopez R, Liebana B, Rojas JL, Gorrochategui J, Sanz MA, Ballesteros J. Pharmacological profiles of acute myeloid leukemia treatments in patient samples by automated flow cytometry: a bridge to individualized medicine. Clin Lymphoma Myeloma Leuk. 2014;14:305–318. doi: 10.1016/j.clml.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 26.Vardiman JW, Harris NL, Brunning RD. The World Health Organization (WHO) classification of the myeloid neoplasms. Blood. 2002;100:2292–302. doi: 10.1182/blood-2002-04-1199. [DOI] [PubMed] [Google Scholar]
- 27.Upton RN, Mould DR. Basic concepts in population modeling, simulation, and model-based drug development: part 3-introduction to pharmacodynamic modeling methods. CPT Pharmacometrics Syst Pharmacol. 2014;3:e88. doi: 10.1038/psp.2013.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beal SL, Sheiner LB, Boeckmann AJ, et al. NONMEM Users Guides. Ellicot City, Maryland: Icon Development Solutions; 1989–2001. [Google Scholar]
- 29.Greco WR, Bravo G, Parsons JC. The search for synergy: a critical review from a response surface perspective. Pharmacol Rev. 1995;47:331–385. [PubMed] [Google Scholar]
- 30.Wood SN. Generalized Additive Models An Introduction with R. Boca Raton, Florida: Chapman & Hall/CRC; 2006. [DOI] [Google Scholar]
- 31.Cheesman S, Shields A. London Cancer North and East. Maximum Anthracycline Doses Guidance. 2016. Available: http://www.londoncancer.org/media/75901/140214-Maximum-Anthracycline-doses-Guideline-v1.pdfm.
- 32.Labar B, Nemet D, Minigo H, Bogdanić V, Jaksić B, Malesević M, Mrsić M. Aclarubicin in the treatment of de-novo acute myelocytic leukaemia. Bone Marrow Transplant. 1989;4(Suppl 3):45–6. [PubMed] [Google Scholar]
- 33.Büchner T, Hiddemann W, Blasius S, Koch P, Maschmeyer G, Tirier C, Sodomann H, Kuse R, Thiel E, Ludwig WD, et al. Adult AML: the role of chemotherapy intensity and duration. Two studies of the AML Cooperative Group. Haematol Blood Transfus. 1990;33:261–6. doi: 10.1007/978-3-642-74643-7_47. [DOI] [PubMed] [Google Scholar]
- 34.Lee JH, Joo YD, Kim H, Bae SH, Kim MK, Zang DY, Lee JL, Lee GW, Lee JH, Park JH, Kim DY, Lee WS, Ryoo HM, Hyun MS, Kim HJ, Min YJ, Jang YE, Lee KH Cooperative Study Group A for Hematology. A randomized trial comparing standard versus high-dose daunorubicin induction in patients with acute myeloid leukemia. Blood. 2011;118:3832–41. doi: 10.1182/blood-2011-06-361410. [DOI] [PubMed] [Google Scholar]
- 35.Ohtake S, Miyawaki S, Kiyoi H, Miyazaki Y, Okumura H, Matsuda S, Nagai T, Kishimoto Y, Okada M, Takahashi M, Handa H, Takeuchi J, Kageyama S, Asou N, Yagasaki F, Maeda Y, Ohnishi K, Naoe T, Ohno R. Randomized trial of response-oriented individualized versus fixed-schedule induction chemotherapy with idarubicin and cytarabine in adult acute myeloid leukemia: the JALSG AML95 study. Int J Hematol. 2010;91:276–83. doi: 10.1007/s12185-009-0480-5. [DOI] [PubMed] [Google Scholar]
- 36.Lee JH, Kim H, Joo YD, Lee WS, Bae SH, Zang DY, Kwon J, Kim MK, Lee J, Lee GW, Lee JH, Choi Y, Kim DY, Hur EH, Lim SN, Lee SM, Ryoo HM, Kim HJ, Hyun MS, Lee KH Cooperative Study Group A for Hematology. Prospective Randomized Comparison of Idarubicin and High-Dose Daunorubicin in Induction Chemotherapy for Newly Diagnosed Acute Myeloid Leukemia. J Clin Oncol. 2017;35(24):2754–63. doi: 10.1200/JCO.2017.72.8618. [DOI] [PubMed] [Google Scholar]
- 37.Choi EJ, Lee JH, Lee JH, Park HS, Ko SH, Hur EH, Moon J, Goo BK, Kim Y, Seol M, Lee YS, Kang YA, Jeon M, Woo JM, Lee KH. Comparison of anthracyclines used for induction chemotherapy in patients with FLT3-ITD-mutated acute myeloid leukemia. Leuk Res. 2018;68:51–6. doi: 10.1016/j.leukres.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 38.Staib P, Staltmeier E, Neurohr K, Cornely O, Reiser M, Schinköthe T. Prediction of individual response to chemotherapy in patients with acute myeloid leukaemia using the chemosensitivity index Ci. Br J Haematol. 2005;128:783–91. doi: 10.1111/j.1365-2141.2005.05402.x. [DOI] [PubMed] [Google Scholar]
- 39.Pemovska T, Kontro M, Yadav B, Edgren H, Eldfors S, Szwajda A, Almusa H, Bespalov MM, Ellonen P, Elonen E, Gjertsen BT, Karjalainen R, Kulesskiy E, Lagström S, Lehto A, Lepistö M, Lundán T, Majumder MM, Marti JM, Mattila P, Murumägi A, Mustjoki S, Palva A, Parsons A, Pirttinen T, Rämet ME, Suvela M, Turunen L, Västrik I, Wolf M, Knowles J, Aittokallio T, Heckman CA, Porkka K, Kallioniemi O, Wennerberg K. Individualized systems medicine strategy to tailor treatments for patients with chemorefractory acute myeloid leukemia. Cancer Discov. 2013;3:1416–29. doi: 10.1158/2159-8290.CD-13-0350. [DOI] [PubMed] [Google Scholar]
- 40.Jun KR, Jang S, Chi HS, Lee KH, Lee JH, Choi SJ, Seo JJ, Moon HN, Im HJ, Park CJ. Relationship between in vitro chemosensitivity assessed with MTT assay and clinical outcomes in 103 patients with acute leukemia. Korean J Lab Med. 2007;27:89–95. doi: 10.3343/kjlm.2007.27.2.89. [DOI] [PubMed] [Google Scholar]
- 41.Pierceall WE, Kornblau SM, Carlson NE, Huang X, Blake N, Lena R, Elashoff M, Konopleva M, Cardone MH, Andreeff M. BH3 profiling discriminates response to cytarabine-based treatment of acute myelogenous leukemia. Mol Cancer Ther. 2013;12:2940–9. doi: 10.1158/1535-7163.MCT-13-0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamada S, Hongo T, Okada S, Watanabe C, Fujii Y, Ohzeki T. Clinical relevance of in vitro chemoresistance in childhood acute myeloid leukemia. Leukemia. 2001;15:1892–7. doi: 10.1038/sj.leu.2402305. [DOI] [PubMed] [Google Scholar]
- 43.Bosanquet AG, Nygren P, Weisenthal LM, et al. Individualized tumor response testing in leukemia and lymphoma. In: Kaspers GJ, Coiffier B, Heinrich MC, et al., editors. Innovative leukemia and lymphoma therapy. New York (NY): Informa Healthcare; 2008. pp. 23–44. [Google Scholar]
- 44.Norgaard JM, Langkjer ST, Palshof T, Pedersen B, Hokland P. Pretreatment leukaemia cell drug resistance is correlated to clinical outcome in acute myeloid leukaemia. Eur J Haematol. 2001;66:160–7. doi: 10.1034/j.1600-0609.2001.00361.x. [DOI] [PubMed] [Google Scholar]
- 45.Sison EA, Brown P. The bone marrow microenvironment and leukemia: biology and therapeutic targeting. Expert Rev Hematol. 2011;4:271–83. doi: 10.1586/ehm.11.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tabe Y, Konopleva M. Role of Microenvironment in Resistance to Therapy in AML. Curr Hematol Malig Rep. 2015;10:96–103. doi: 10.1007/s11899-015-0253-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zahreddine H, Borden KL. Mechanisms and insights into drug resistance in cancer. Front Pharmacol. 2013;4:28. doi: 10.3389/fphar.2013.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li ZW, Dalton WS. Tumor microenvironment and drug resistance in hematologic malignancies. Blood Rev. 2006;20:333–42. doi: 10.1016/j.blre.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 49.Quartino A, Karlsson MO, Freijs A, Jonsson N, Nygren P, Kristensen J, Lindhagen E, Larsson R. Modeling of in vitro drug activity and prediction of clinical outcome in acute myeloid leukemia. J Clin Pharmacol. 2007;47:1014–21. doi: 10.1177/0091270007302563. [DOI] [PubMed] [Google Scholar]
- 50.Martínez-Cuadrón D, Gil C, Serrano J, Rodríguez G, Pérez-Oteyza J, García-Boyero R, Jiménez-Bravo S, Vives S, Vidriales MB, Lavilla E, Pérez-Simón JA, Tormo M, Colorado M, Bergua J, López JA, Herrera P, Hernández-Campo P, Gorrochategui J, Primo D, Rojas JL, Villoria J, Moscardó F, Troconiz I, Linares Gómez M, Martínez-López J, Ballesteros J, Sanz M, Montesinos P Spanish PETHEMA group. A precision medicine test predicts clinical response after idarubicin and cytarabine induction therapy in AML patients. Leuk Res. 2018;76:1–10. doi: 10.1016/j.leukres.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 51.Kim HP, Gerhard B, Harasym TO, Mayer LD, Hogge DE. Liposomal encapsulation of a synergistic molar ratio of cytarabine and daunorubicin enhances selective toxicity for acute myeloid leukemia progenitors as compared to analogous normal hematopoietic cells. Exp Hematol. 2011;39:741–50. doi: 10.1016/j.exphem.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 52.Lancet JE, Cortes JE, Hogge DE, Tallman MS, Kovacsovics TJ, Damon LE, Komrokji R, Solomon SR, Kolitz JE, Cooper M, Yeager AM, Louie AC, Feldman EJ. Phase 2 trial of CPX-351, a fixed 5:1 molar ratio of cytarabine/daunorubicin, vs cytarabine/daunorubicin in older adults with untreated AML. Blood. 2014;123:3239–46. doi: 10.1182/blood-2013-12-540971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Megías-Vericat JE, Martínez-Cuadrón D, Sanz MA, Montesinos P. Salvage regimens using conventional chemotherapy agents for relapsed/refractory adult AML patients: a systematic literature review. Ann Hematol. 2018;97:1115–53. doi: 10.1007/s00277-018-3304-y. [DOI] [PubMed] [Google Scholar]