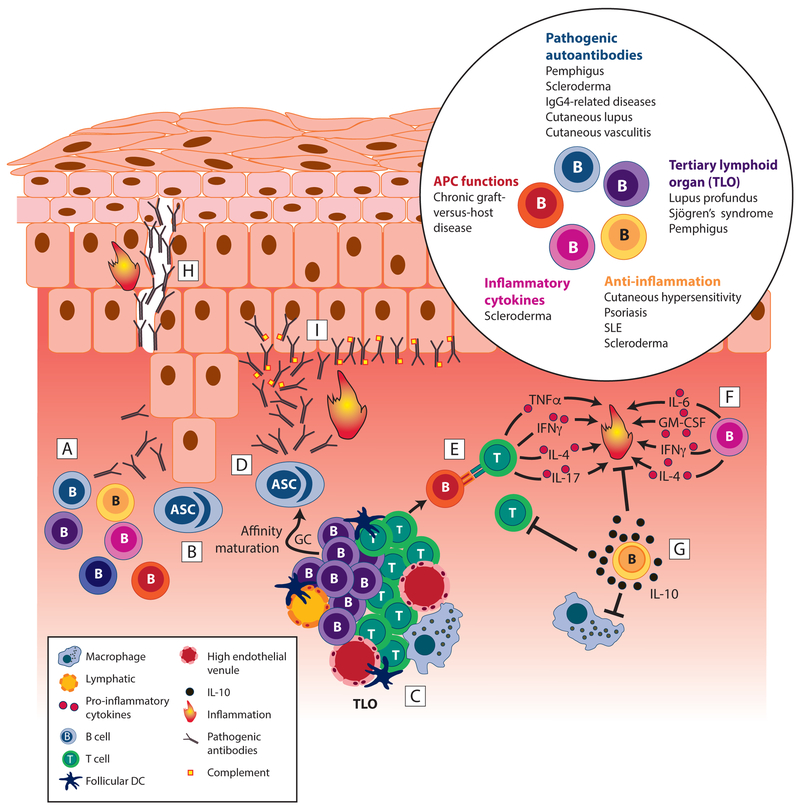
Figure 2. Skin-associated B cells are critical to driving and suppressing inflammation.
Skin-associated B cells play several important roles in skin inflammation. [A] B cell subsets with various functions accumulate in inflamed skin. [B] Antibody-secreting cells accumulate in inflamed skin and secrete antibodies with reactivity to cutaneous antigens, including autoantigens and skin-associated allergens. [C] Aggregates of collaborating B and T cells form, and B cells initiate further development of tertiary lymphoid organs that support activation and differentiation of pathogenic B and T cells. [D] High affinity autoreactive plasma cells and IgGs emerge from GC reaction in TLO, exacerbating inflammation. [E] B cells act as APCs inducing T cell activation and pro-inflammatory cytokine production. [F] B cells produce pro-inflammatory cytokines, like IL-6, and potentially GM-CSF, IFN-γ, or IL-4, all of which promote local inflammation and, in case of IL-6, fibrosis via stimulation of fibroblasts. [G] B cells secrete IL-10 that suppresses activation of other leukocytes, including T cells and macrophages, thereby counteracting inflammation. [H] Pathogenic autoantibody deposition can destroy cell-cell junctions creating acantholysis or gaps between cells as in pemphigus. [I] Autoantibodies can accumulate at the dermoepidermal junction as in cutaneous lupus erythematosus, leading to complement activation and other inflammatory downstream effector functions. Inset, Summary of disease settings in which skin-associated B cells modulate skin inflammation. ASC, antibody secreting cell; SLE, systemic lupus erythematosus. GC, germinal center; TLO, tertiary lymphoid organ.