Abstract
In cardiac myocytes Calmodulin (CaM) bound to the ryanodine receptor (RyR2) constitutes a large pool of total myocyte CaM, but the CaM-RyR2 affinity is reduced in pathological conditions. Knock-in mice expressing RyR2 unable to bind CaM also developed hypertrophy and early death. However, it is unknown whether CaM released from this RyR2-bound pool participates in pathological cardiac hypertrophy.
We found that angiotensin II (AngII) or phenylephrine (PE) both cause CaM to dissociate from the RyR2 and translocate to the nucleus. To test whether this nuclear CaM accumulation depends on CaM released from RyR2, we enhanced CaM-RyR2 binding affinity (with dantrolene), or caused CaM dissociation from RyR2 (using suramin). Dantrolene dramatically reduced AngII- and PE-induced nuclear CaM accumulation. Conversely, suramin enhanced nuclear CaM accumulation. This is consistent with nuclear CaM accumulation coming largely from the CaM-RyR2 pool. CaM lacks a nuclear localization signal (NLS), but G-protein coupled receptor kinase 5 (GRK5) binds CaM, has a NLS and translocates like CaM in response to AngII or PE. Suramin also promoted GRK5 nuclear import, and caused nuclear export of histone deacetylase 5 (HDAC5). Dantrolene prevented these effects. After 2–8 weeks of pressure overload (TAC) CaM binding to RyR2 was reduced, nuclear CaM and GRK5 were both elevated and there was enhanced nuclear export of HDAC5.
Stress (acute AngII or TAC) causes CaM dissociation from RyR2 and translocation to the nucleus with GRK5 with parallel HDAC5 nuclear export. Thus CaM dissociation from RyR2 may be an important step in driving pathological hypertrophic gene transcription.
Keywords: ryanodine receptor, calmodulin, dantrolene, GRK5, HDAC5
1. Introduction
Cardiac pathological hypertrophy occurs in response to pressure overload, such as chronic hypertension, aortic stenosis, aortic constriction, and myocardial infarction. The heart initially compensates for the overload by hypertrophic growth, but function in the pathological hypertrophied heart eventually decompensates and, finally, leads to heart failure (HF). Thus cardiac pathological hypertrophy is an important risk factor for cardiovascular disease [1].
Calmodulin (CaM) is a ubiquitous Ca2+ binding protein that binds to ryanodine receptor type2 (RyR2) even at diastolic [Ca2+]i and modulates its channel function [2]. CaM inhibits RyR2 open probability at physiological [Ca2+] by binding to a CaM binding region (aa 3583–3603) of RyR2 [3,4], suggesting that the RyR2-bound CaM (CaM-RyR2) stabilizes the closed state of RyR2 channels in the resting state of normal cells. Several reports have suggested that defective CaM-RyR2 interaction becomes a pathogenic factor in heart disease. We recently found that the CaM binding affinity to RyR2 is reduced in HF [5–7] or in knock-in (KI) mice carrying a human CPVT-associated RyR2 mutation (R2474S) [8,9] or by oxidation [10] or in chronic pressure overload (induced by transverse aortic constriction (TAC)) [11], and that lower CaM binding promoted arrhythmogenic SR Ca2+ leak. Moreover, Yamaguchi et al. [12] reported that KI mice, in which RyR2 is unable to bind CaM, developed hypertrophy and had an early death. While CaM binding to RyR2 is known to importantly regulate RyR2 gating in cardiac myocytes, the role of CaM released from RyR2 is unknown. Ai et al. [13] reported that the level of CaM associated with RyR2 was decreased in rabbit HF, despite unaltered total CaM expression in HF. In hippocampal or cortical neurons, Ca2+ or Ca2+/CaM-dependent protein kinase II (CaMKII) caused translocation of CaM from the cytoplasm to the nucleus [14,15], although only 5% of total endogenous CaM was free in the cytoplasm. In resting cardiac ventricular myocytes only ~1% of total CaM is freely diffusible [16] and bound CaM is highly concentrated along Z-lines where >90% of that CaM is RyR2-bound [5]. These reports prompted us to test whether the nuclear accumulation of CaM in myocytes coincides with and depends upon loss of CaM-binding to RyR2.
G protein-coupled receptor kinases (GRKs), notably GRK5, which is most abundant in the heart, are crucial in regulating cardiac hypertrophy. GRK5 nuclear accumulation accelerated cardiac hypertrophy and HF in the TAC model [17]. GRK5 has CaM binding sites at each terminal domain, with the N-terminal CaM binding site of GRK5 being most critical [18]. Gold et al. [19] showed that mutant GRK5 unable to bind CaM at its N-terminal CaM binding site displayed less nuclear accumulation during neurohumoral stimuli, suggesting that GRK5 translocation to the nucleus is dependent on, and absolutely requires, CaM binding to GRK5 at its specific N-terminal CaM binding site. However, again, the source of CaM, which binds to GRK5, is unknown.
Class II nuclear histone deacetylases (HDACs 4,5,7 and 9), especially HDAC5, normally represses hypertrophic transcriptional activation. However, HDAC5 phosphorylation by GRK5, protein kinase D or CaMKII in response to neurohumoral stimuli and HF drives nuclear export of HDAC5 and promotes a hypertrophic program of cardiac gene expression [17,20–23].
Here we investigate whether CaM coming from the RyR2-CaM pool can migrate to the nucleus with GRK5 and drive HDAC5 nuclear export and hypertrophic signaling. We used angiotensin II (Ang II) and phenylephrine (PE), which are physiological hypertrophic agonists, and performed immunofluorescence to assess the movement of endogenous CaM, GRK5 and HDAC5 in the cardiomyocytes. Furthermore, we used dantrolene, which enhances CaM-RyR2 affinity and stabilizes the RyR2 channel gating [7–10]. Since suramin directly binds to the core RyR CaM binding site and rapidly releases CaM from the RyR2 [5,24], we also used suramin to release CaM binding to RyR2 and thereby test the hypothesis that release of CaM from RyR could by itself drive nuclear CaM translocation. Moreover, TAC-induced hypertrophy and HF was examined at 2 and 8 weeks, respectively, to evaluate the RyR2-CaM movement.
2. Materials and methods
Adult mouse ventricular myocytes were isolated and permeabilized as previously described [9]. All procedures were performed according to the Guiding Principles in the Care and Use of the Animals approved by Guidelines of the Animal Ethics Committee of Yamaguchi University School of Medicine. All experiments were performed at room temperature (25°C) and data are given as mean ± SEM of 20–80 cells from 3–4 hearts. To quantify the fluorescence intensity based on confocal microscopy, we measured a ratio of nuclear CaM fluorescence to DAPI or ratio of CaM-RyR2 fluorescence to RyR2 fluorescence or, for GRK5 and HDACs, a ration of nuclear fluorescence to cytoplasmic fluorescence were used. An expanded Methods section can be found in the Online Data Supplement.
3. Results
3.1. CaM moves from RyR2 to nucleus after treatment with hypertrophic agonists or suramin, but is prevented by dantrolene
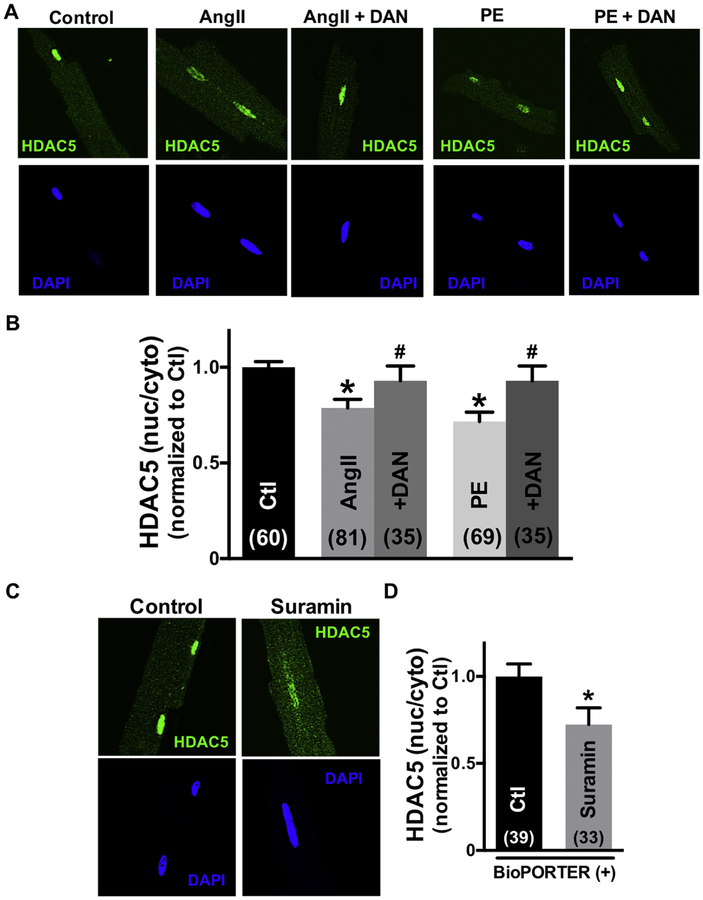
First, we tested whether endogenous CaM concentrates at Z-line near the RyR2 by using antibodies for CaM (EP799Y, Abcam) and RyR (C3–33, Sigma-Aldrich). We also treated saponin-permeablized myocytes with suramin to rapidly and completely release CaM from RyR2. Online Figure I shows that both endogenous RyR2 and CaM are concentrated at Z-lines. Treatment with suramin (which releases CaM from RyR2) strongly suppresses the Z-line CaM signal (Online Figure II). These results confirm that this anti-CaM antibody recognizes endogenous CaM and that suramin releases CaM from the RyR at the Z-line.
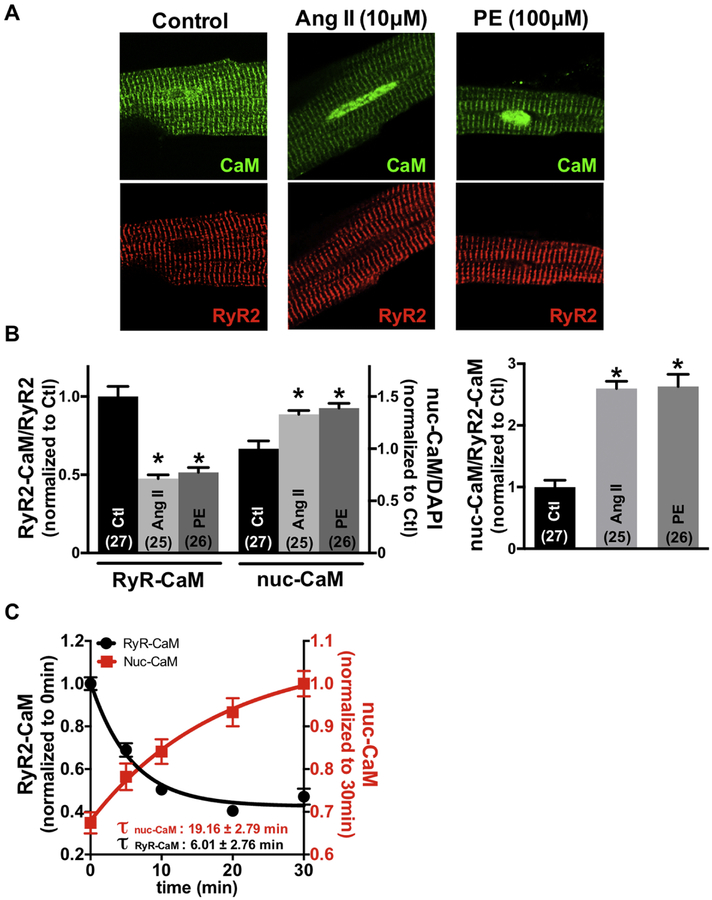
In adult mouse ventricular myocytes CaM is highly concentrated at the Z-line (Fig 1A, left) where >90% of that CaM is specifically bound to RyR2 [5]. After treating intact resting myocytes with 10 μM AngII or 100 μM PE (for 30 min), CaM was reduced at the RyR2, but increased in the nucleus (Fig 1B). We also measured the kinetics of AngII-induced CaM translocation. The time constant of CaM loss at the RyR2 (τwash-out = 6.01 ± 2.76 min) was faster than the rise in nuclear CaM accumulation (τwash-in = 19.16 ± 2.79 min), which would be consistent with CaM dissociation from RyR2 as a precursor for nuclear CaM accumulation (Fig 1C). The more gradual rise in nuclear [CaM] is likely the result of translocation of CaM, potentially released from the RyR2 to travel from regions distant from the nucleus.
Fig 1.
Angiotensin II (Ang II) or phenylephrine (PE) induces calmodulin (CaM) movement from RyR2 to nucleus. (A) Immunofluorescent images of adult cardiomyocytes during control, 30min exposure to 10 μmol/L Ang II, or 100 μmol/L PE. RyR2 (red) and CaM (green) are shown. (B) Summary of CaM translocation treatment with Ang II and PE corresponding to the experiment in panel A. (C) Kinetics of CaM movements of RyR2 binding CaM and nucleus CaM treated with 10 μmol/L Ang II. Data are mean ± SE, n values on bars, *P<0.05 vs. Control (Ctl) (one-way ANOVA followed by tukey-kramer’s post-test).
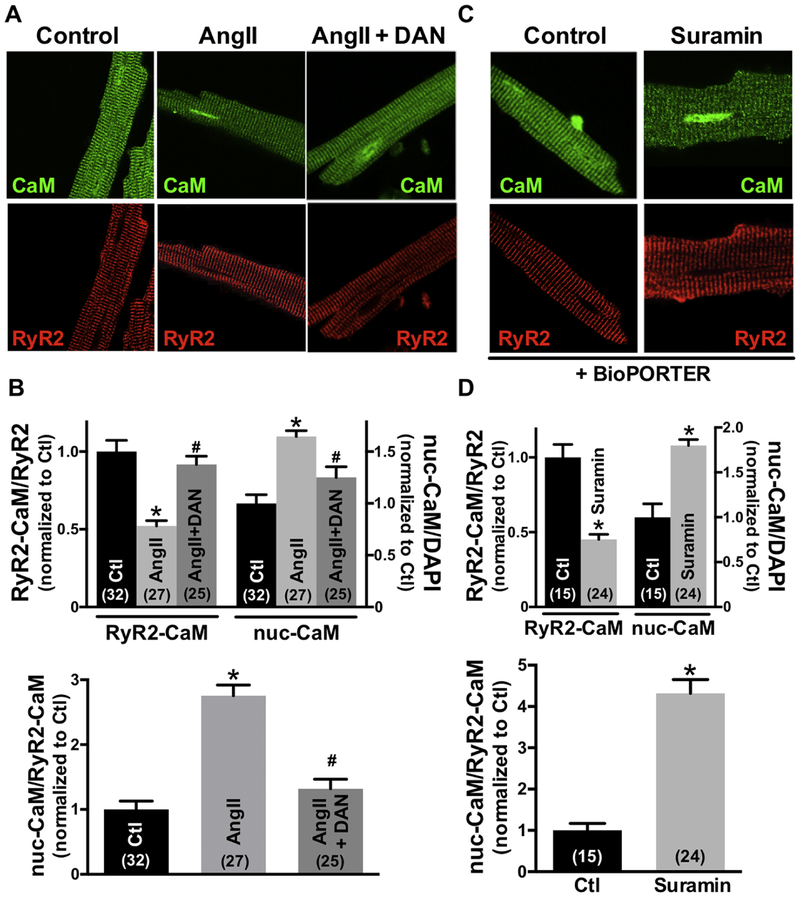
To further test whether this CaM accumulated in the cell nuclei comes from RyR2-CaM, we treated the cells with dantrolene (DAN), which strengthens CaM binding to RyR2, thus stabilizing RyR2 channel gating. Fig 2A shows confocal immunofluorescence images of adult cardiomyocytes with RyR2 and CaM co-localization and CaM translocation to the nucleus during exposure to 10 μM AngII. Treatment with dantrolene prevented the AngII-induced loss of CaM at the RyR2 and also prevented nuclear CaM accumulation, suggesting that nuclear CaM accumulation comes from RyR2-CaM (Fig 2B). To test whether release of CaM from the RyR2 was sufficient by itself to drive CaM to the nucleus, we measured the Z-line CaM and nuclear CaM fluorescence intensity upon suramin exposure (which rapidly dissociates CaM from the RyR2 [5,24]). Since suramin is not cell permeable, 100 μM suramin was introduced to intact cardiomyocytes using the protein delivery reagent, BioPORTER, and the Z-line and nuclear endogenous CaM were assessed by immunofluorescence analysis (Fig 2C). As shown in Fig 2D, suramin significantly reduced the [CaM] at RyR2 and increased the nuclear [CaM]. We also plotted nuclear-CaM fluorescence intensity versus RyR2-CaM fluorescence intensity for the same intact cardiomyocytes. The dependence of nuclear-CaM fluorescence on RyR2-CaM fluorescence was fit by a linear regression, consistent with RyR2-CaM being translocated to the nucleus (Online Figure III). Taken together, the data support the view that stimulation of myocytes with hypertrophic agonists (Ang II, PE) or the CaM-RyR2 disruptor suramin, can reduce CaM at the RyR2 and promote nuclear CaM accumulation. Conversely, dantrolene prevents the AngII-induced loss of RyR2-CaM and nuclear CaM accumulation. These findings suggest that the source of nuclear CaM accumulation is RyR2-bound CaM.
Fig 2.
Dantrolene (DAN) restores CaM binding to RyR2 and prevents nuclear CaM accumulation. Conversely, treatment with suramin reduces the RyR2-CaM binding and promotes the nuclear CaM accumulation. (A), (C) Immunofluorescent images of adult cardiomyocytes during control, 30min exposure to 10 μmol/L Ang II with or without DAN, and suramin treatment with protein delivery reagent for 3hours. RyR2 (red) and CaM (green) are shown. (B) (D) Summary of CaM translocation treatment with Ang II, Ang II+DAN, or suramin. Data are mean ± SE, n values on bars, *P<0.05 vs. Control (Ctl), #P<0.05 vs. Ang II (one-way ANOVA followed by tukey-kramer’s post-test; Fig 2B, unpaired Student’s t-test; Fig 2D).
3.2. Angiotensin II-induced redox modification reduces RyR2-CaM binding
Next, we explored the reason for the Ang II-mediated reduction of CaM binding to RyR2. We previously showed that increased reactive oxygen species (ROS) pathologically alters the RyR2 structure (domain interaction between N-terminal and central domains) and that this conformation (defective domain interaction) reduces the RyR2-CaM binding affinity [10]. To monitor intracellular ROS level, we used CellROX, an oxidative stress detection fluorogenic probe. Online Figure IVA shows confocal CellROX fluorescence images in control or Ang II-treated myocytes with or without dantrolene. As shown in Online Figure IVB, the intracellular ROS level was significantly increased after addition of 10 μM AngII, and dantrolene did not alter the intracellular oxidative stress level overall. Thus, AngII elevates ROS which might mediate the AngII-induced reduction in RyR2-CaM binding affinity. Dantrolene did not alter the AngII-induced rise in ROS, indicating that the dantrolene’s effect to limit CaM movement from RyR2 to the nucleus (Fig 2B) is downstream of AngII-induced ROS.
3.3. GRK5 participates in CaM nuclear translocation
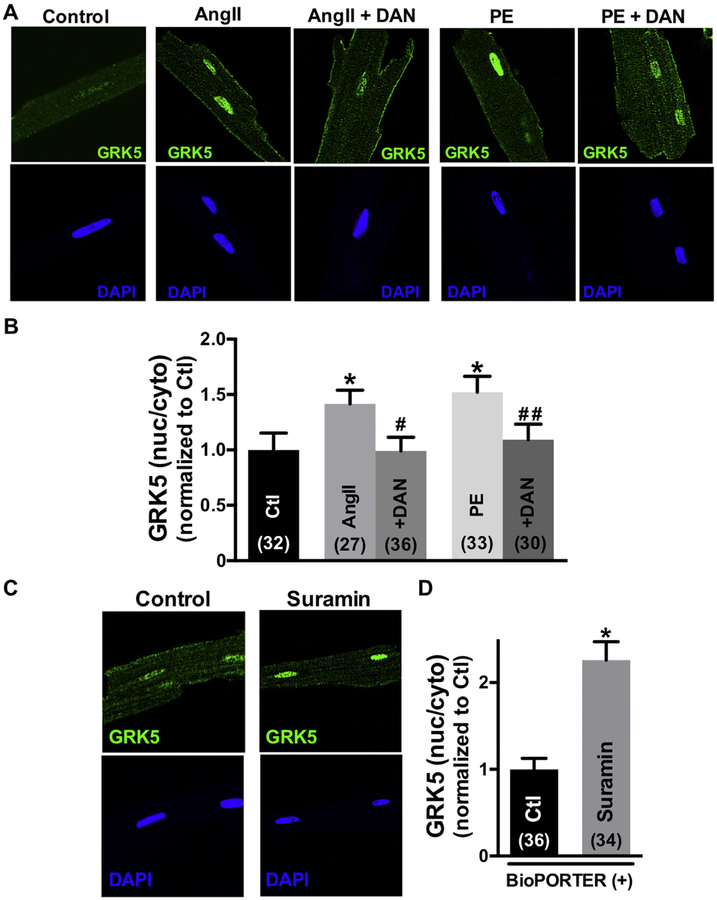
Since CaM itself lacks a nuclear localization sequence (NLS), but GRK5 has both CaM binding sites as well as an NLS, we tested whether GRK5 might mediate the CaM translocation from RyR2 to nucleus induced by AngII or PE. Fig 3A shows confocal immunofluorescence images of GRK5 and nuclei stained with 4’,6-diamidino-2-phenylindole (DAPI) in intact cardiomyocytes that are untreated (control), or treated with AngII, PE or AngII (± DAN). AngII and PE both induced translocation of GRK5 from the cytosol to the nucleus, but treatment with dantrolene prevented this AngII- and PE-induced GRK5 movement (Fig 3B). Thus, just like CaM, GRK5 also moves to the nucleus in response to AngII and PE, and is also prevented by dantrolene (which promotes CaM-RyR2 binding). Directly displacing CaM from RyR2 with suramin (as in Fig 2) also promoted GRK5 nuclear translocation, even in the absence of AngII or PE (Fig 3C–D).
Fig 3.
Angiotensin II (Ang II), phenylephrine (PE) or suramin induce GRK5 nuclear accumulation, but dantrolene (DAN) prevents this. (A), (C) Immunofluorescent images of GRK5 and nuclei (DAPI) in adult cardiomyocytes during control, 30min exposure to 10 μmol/L Ang II, Ang II+DAN, or 3hours exposure to suramin by protein delivery reagent. (B), (D) Summary of GRK5 translocation treatment with Ang II and PE with or without DAN, or suramin. Data are mean ± SE, n values on bars, *P<0.05 vs. control (Ctl), #P<0.05 vs. Ang II, ##P<0.05 vs. PE (one-way ANOVA followed by tukey-kramer’s post-test; Fig 3B, unpaired Student’s t-test; Fig 3D).
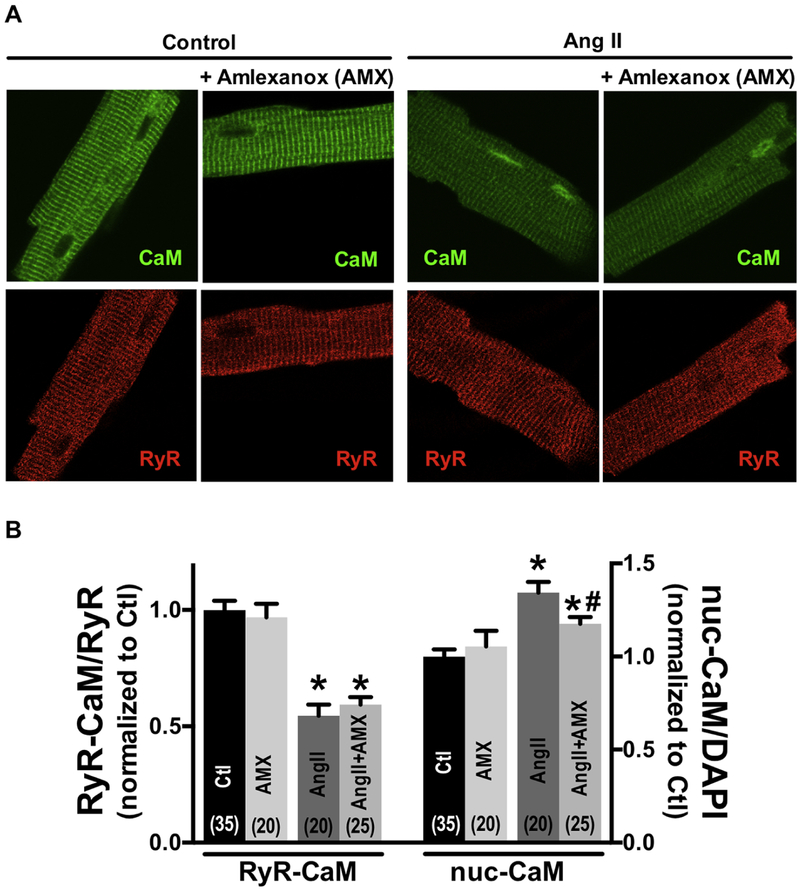
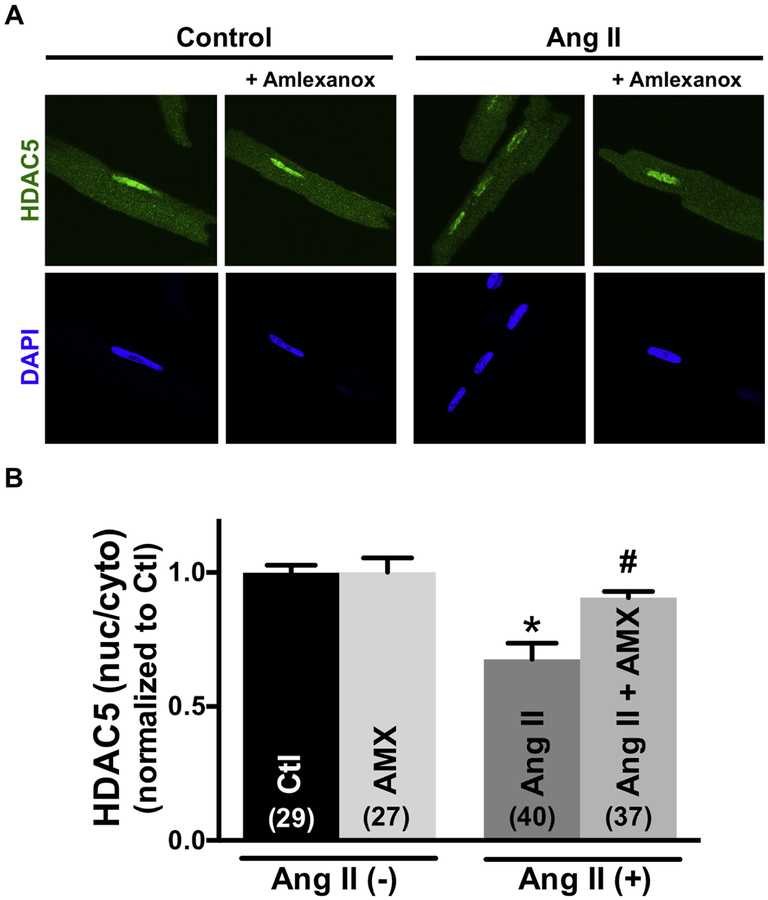
To test whether CaM translocation to the nucleus required GRK5 enzymatic activity, we used the GRK5 inhibitor amlexanox (AMX), a pharmacological inhibitor of GRK5 kinase activity [25]. At baseline 50 μM AMX did not alter nuclear GRK5 levels, but it prevented the AngII-induced nuclear GRK5 accumulation (Online Figure V). This confirms that AMX inhibits the AngII-induced GRK5 mobilization (and that GRK5 enzymatic activity is required for its mobilization). AMX did not alter basal CaM distribution between RyR2 or nucleus, nor the AngII-induced release of CaM from the RyR2 (Fig 4). However, AMX treatment significantly decreased the AngII-induced nuclear CaM accumulation compared with treatment with AngII alone (Fig 4A–B). These results suggest that CaM translocation is enhanced by GRK5 activity.
Fig 4.
Amlexanox (AMX) prevents calmodulin (CaM) nuclear accumulation, but not effect on Angiotensin II (Ang II) induced CaM-RyR2 dissociation. (A) Immunofluorescent images of CaM and nuclei (DAPI) in adult cardiomyocytes during control, 30min exposure to 50 μmol/L Amlexanox, 10 μmol/L Ang II, Ang II+50 μmol/L Amlexanox. (B) Summary of CaM translocation treatment with Ang II or Ang II+Amlexanox. Data are mean ± SE, n values on bars, *P<0.05 vs. control (Ctl), #P<0.05 vs. Ang II (one-way ANOVA followed by tukey-kramer’s post-test).
3.4. AngII, PE and suramin induced HDAC5 nuclear export is inhibited by dantrolene
Next, we tested whether CaM release from RyR2 is critical for the AngII- or PE-induced HDAC5 nuclear export that drives pathological cardiac hypertrophic signaling. Fig 5A shows immunofluorescent images of HDAC5 and nuclei stained by DAPI in myocytes in the absence and presence of Ang II or PE (±dantrolene). AngII and PE both induced the expected HDAC5 nuclear export, but this was significantly suppressed when dantrolene was used to stabilize CaM-RyR2 binding (Fig 5B). AngII and PE also promote nuclear export of HDAC4 (another class IIa HDAC), but again that nuclear export was prevented by dantrolene (Online Figure VIA–B). Additionally, displacing CaM from RyR2 with suramin induced HDAC5 nuclear export, even in the absence of AngII or PE (Fig 5C–D). Furthermore, inhibition of GRK5 with amlexanox alone did not alter HDAC5 nuclear export, but it suppressed the AngII-induced HDAC5 nuclear vs. AngII alone (Fig 6A–B). The collective results suggest that depletion of CaM from RyR2 and nuclear accumulation of CaM requires GRK5 activity for AngII-induced and HDAC5-dependent pathological cardiac hypertrophic signaling.
Fig 5.
Angiotensin II (Ang II), phenylephrine (PE) or suramin induce HDAC5 nuclear export but dantrolene (DAN) restores. (A), (C) Immunofluorescent images of HDAC5 and nuclei (DAPI) in adult cardiomyocytes under conditions of after 30min exposure to 10 μmol/L Ang II, Ang II+DAN, or 3hours exposure to suramin introduced using protein delivery reagent. (B), (D) Summary of HDAC5 translocation treatment with Ang II, PE with or without DAN, or suramin. Data are mean ± SE, n values on bars, *P<0.05 vs. control (Ctl), #P<0.05 vs. Ang II, ##P<0.05 vs. PE (one-way ANOVA followed by tukey-kramer’s post-test; Fig 3B, unpaired Student’s t-test; Fig 3D).
Fig 6.
Amlexanox prevents HDAC5 nuclear exports, which were caused by Angiotensin II (Ang II). (A) Immunofluorescent images of HDAC5 and nuclei (DAPI) in adult cardiomyocytes during control, 30min exposure to 50 μmol/L Amlexanox, 10 μmol/L Ang II, Ang II+50 μmol/L Amlexanox. (B) Summary of HDAC5 nuclear exports treatment with Ang II or Ang II+Amlexanox. Data are mean ± SE, n values on bars, *P<0.05 vs. control (Ctl), #P<0.05 vs. Ang II (one-way ANOVA followed by tukey-kramer’s post-test).
3.5. Pressure overload causes CaM movement from RyR2 to nucleus with GRK5 and promotes the nuclear export of both HDAC5 and HDAC4
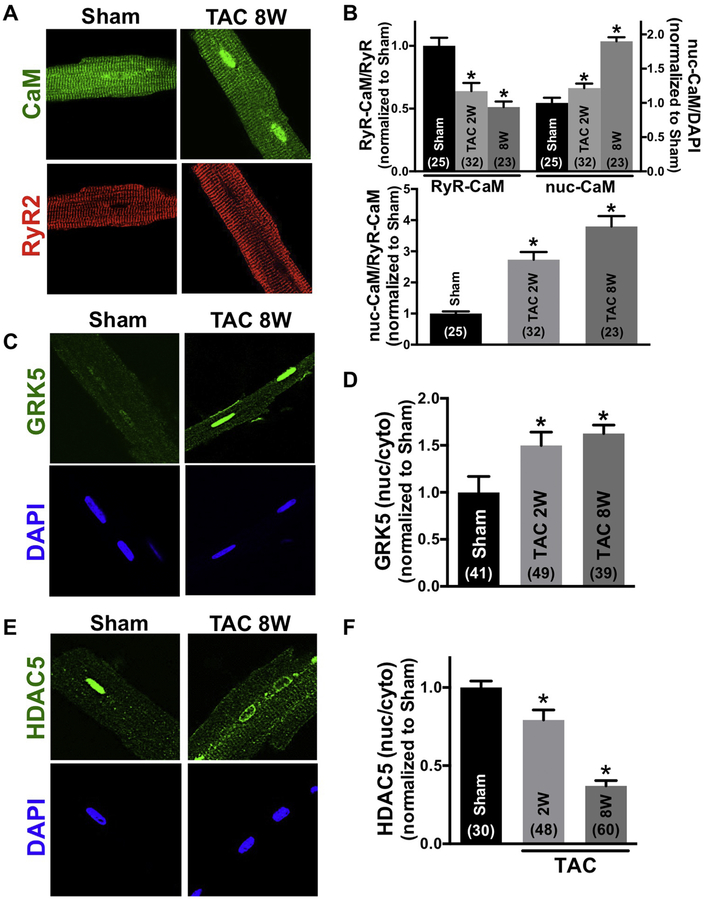
To test the relevance of this RyR2-CaM GRK5 translocation pathway to hypertrophy and HF we used a classic chronic pressure overload model of transverse aortic constriction (TAC) in 12-week-old mice, during both the early hypertrophic phase (2 weeks post-TAC) and HF phase (8 weeks post-TAC). Online Figure VIIA–C shows that left ventricular and septal wall thickness was significantly increased 2 post-TAC with only slight reduction in fractional shortening. However, at 8 weeks post-TAC LV fractional shortening was dramatically reduced (consistent with hypertrophic and HF monikers). Two weeks post-TAC we observed a significant shift of CaM from RyR2 to nucleus, and that was more pronounced 8 weeks post-TAC (Fig 7A–B). GRK5 also moved significantly to the nucleus at both 2 and 8 weeks post-TAC, although this was not significantly different between hypertrophy and HF stages (Fig 7C–D). Taken together, these results suggest that 8 weeks after TAC (HF phase), there may be a signal cascade other than GRK5 that drives or retains CaM in the nucleus. HDAC5 (and HDAC4) also shifted out of the nucleus significantly at 2 weeks post-TAC and even more dramatically at 8 weeks post-TAC (Fig 7E–F and Online Figure VIC–D). Thus, while CaM and GRK5 acutely translocate to the nucleus upon hypertrophic Gq-coupled receptor activation, chronic nuclear accumulation (or retention) of CaM correlates best with HDAC5 and HDAC4 nuclear export in the HF phase.
Fig 7.
CaM, GRK5 and HDAC5 translocation of chronic pressure overload (TAC) mouse. (A), (C), (E) Immunofluorescent images of CaM and RyR2, GRK5, HDAC5 and nuclei (DAPI) in sham and TAC cardiomyocytes. After TAC (vs. sham), the binding affinity of CaM to RyR2 was lower and nuclear CaM was elevated along with GRK5. HDAC5 nuclear export increased in TAC model. (B), (D), (F) Summary of CaM, GRK5, HDAC5 translocation in 2, 8 weeks TAC model. Data are mean ± SE, n values on bars, *P<0.05 vs. sham (one-way ANOVA followed by dunnet’s post-test).
4. Discussion
Prior work showed that CaM binding to the amino terminal of GRK5 is required for AngII and PE-induced nuclear translocation in hypertrophic transcriptional signaling, but the source of CaM was not identified [19]. In another sphere, work has shown that pathological signals cause reduced RyR2 CaM binding and acute arrhythmogenic SR Ca2+ leak, but have not explored potential AngII, PE or nuclear consequences [5–13]. Here we demonstrate a novel direct mechanistic connection between these HF and arrhythmia fields. That is, AngII, PE and pressure overload trigger CaM release from RyR2 (arrhythmogenic itself) that is both necessary and sufficient to drive nuclear translocation of CaM with GRK5 and kinase-dependent nuclear export of hypertrophy/HF-related transcriptional regulators HDAC4 and 5 (Fig 8).
Fig 8.
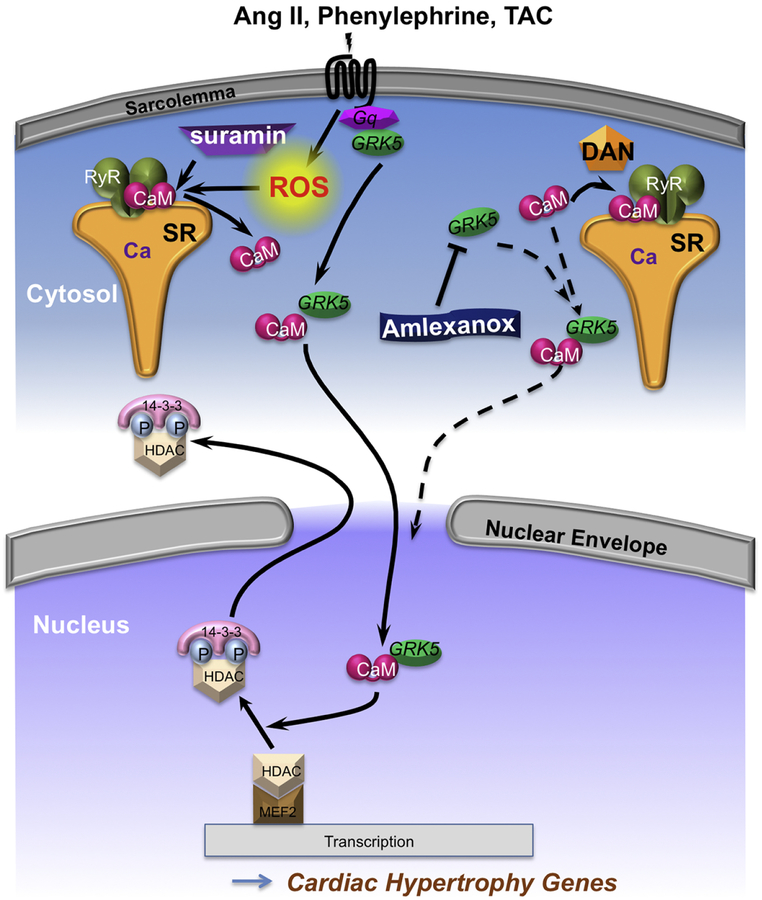
Cartoon illustrating the CaM, GRK5 and class IIa HDACs translocation at pathological condition. Under stress (Ang II, PE or TAC), which increases the ROS level in the myocytes, CaM dissociates from RyR2 and translocation to the nucleus with GRK5 and causes nuclear export of class IIa HDACs. Treatment with suramin, which completely knock off the CaM from RyR2, promotes nuclear CaM accumulation together with GRK5 and there was enhanced nuclear export of class IIa HDACs. Conversely, dantrolene, RyR stabilizer, and amlexanox, GRK5 inhibitor, prevent the both CaM and GRK5 nuclear accumulation and suppressed the class IIa HDACs nuclear export. Our findings suggesting that dissociation of CaM from RyR2 drives CaM to the nucleus, together with GRK5 with parallel class IIa HDACs nuclear export, as a potentially important step driving pathological cardiac hypertrophy. DAN: dantrolene.
Several key novel findings were made, all in adult ventricular myocytes. First, AngII or PE causes ROS-dependent reduction of CaM-RyR binding. Second, CaM which dissociates from the RyR2, translocates to the nucleus together with GRK5, and drives nuclear export of class IIa HDACs, thereby derepressing pathological cardiac hypertrophic genes transcription. Third, this AngII and PE-driven cascade can be inhibited by stabilizing CaM-RyR with dantrolene, and can be mimicked by direct displacement of CaM from RyR2 by suramin. Fourth, direct inhibition of GRK5 activity using amlexanox [25] prevents nuclear CaM accumulation, but does not prevent AngII-induced CaM release from RyR2, suggesting that GRK5 activity is critical in nuclear CaM (and GRK5) accumulation. Fifth, in physiological hypertrophy and HF after TAC, we see this same CaM dissociation from RyR2, nuclear accumulation of CaM with GRK5 and class IIa HDAC nuclear export. Our data support a working model (Fig 8) in which CaM released from the CaM-RyR2 pool is obligatory in co-migration with GRK5 to the nucleus where it promotes class IIa HDAC nuclear export, and that this is an important step in pathological cardiac hypertrophy leading to HF.
4.1. CaM binding at an RyR2-bound pool provides a source of CaM for nuclear import
CaM has many cellular targets, and signaling via CaM is critical in myocyte Ca2+-handling, electrophysiology, hypertrophy and HF signaling [26–28]. CaM binding directly to the RyR2 stabilizes RyR2 gating, and constitutes 15–20% of total myocyte CaM [15]. Detailed intermolecular FRET studies demonstrated that >90% of the prominent Z-line localized CaM (visible here in Fig 1, 2, 4 and 7) is specifically bound to RyR2 (and suppresses diastolic SR Ca2+ leak)[5]. Thus any reduction in Z-line CaM binding is indicative of reduced RyR2 binding (confirmed here for antibody detection of endogenous Z-line CaM that was suramin-sensitive; Online Figure I-II). Even during diastole 99% of all myocyte CaM is bound to cellular proteins, such that in the cytosol only 1% (60 nM) is available to move freely and many targets may compete for free CaM [16]. Ventricular myocyte CaM expression is unaltered in HF [13], but CaM binding affinity to RyR2 is reduced in several models of HF [5–7], CPVT [8,9], oxidative RyR2 stress [10], or RyR2 phosphorylation by CaMKII [7]. So the RyR2 constitutes a major pool (or source) of CaM that may be mobilized and influence other targets. While the AngII-induced ROS increase would suffice to release CaM from RyR2 [10], ROS can also promote local autonomous CaMKII activation [7] which may further promote this effect. Reduction of RyR2-CaM binding by any of the above causes is known to promote arrhythmogenic diastolic SR Ca2+ release [5–11], but the CaM destination following RyR2 dissociation is unknown.
One novel finding here was that the physiological hypertrophic agonists AngII and PE (known to be pivotal in left ventricular hypertrophy [29] and increase risks of HF, stroke, and arrhythmias [30]) acutely reduces CaM-RyR2 binding and enhances nuclear CaM accumulation. Endothelin-1 could also do this in cultured neonatal cardiomyocytes [31]. To test whether RyR2-bound CaM was the source of CaM accumulating in the nucleus, we (1) compared the kinetics of both processes, (2) selectively stabilized RyR2-CaM binding by dantrolene [7–10], and (3) displaced RyR2-bound CaM with suramin [5,24]. CaM was released from the RyR2 faster than its accumulation in the nucleus (Fig 1C), making RyR2 a plausible source of CaM for nuclear accumulation. Preventing AngII-induced CaM release from RyR2 (as shown directly for ROS [10]) also inhibited AngII-induced nuclear accumulation (Fig 2). Conversely, displacement of RyR2-bound CaM by suramin promoted nuclear CaM accumulation, even without AngII treatment. We conclude that CaM released from the RyR2-bound pool is a major source of CaM that is driven into the nucleus by AngII and PE. While CaM could simply diffuse into the nucleus, it lacks an NLS and could bind to many other myocyte targets. This raises questions as to how and if CaM is directed to the nucleus by partnering with a specific NLS bearing target.
4.2. GRK5 participates in CaM nuclear translocation
GRK5 contains a NLS [32] and two CaM binding domains [18,19,33]. Gold et al. [19] showed that both AngII and PE induced sarcolemmal GRK5 translocation to the nucleus in adult ventricular myocytes over a similar time course as shown here for CaM. They also showed that mutation of the N-terminal CaM-binding domain of GRK5 was sufficient to prevent GRK5 nuclear translocation in both cultured neonatal and adult ventricular myocytes [19]. However, neither CaM translocation, GRK5 activity nor the source of CaM that promoted GRK5 translocation was examined. We now show that the AngII- or PE-induced GRK5 nuclear accumulation requires CaM released from RyR2 (prevented by dantrolene), and can even be mimicked by forced release of CaM from RyR2 by suramin (Fig 3A–C). Moreover, amlexanox inhibition of GRK5 kinase activity (and its myocyte enhancer factor 2; MEF2 transcriptional activation [25]) suppresses the AngII-induced nuclear CaM accumulation, despite retained CaM release from RyR2 (Fig 4). Thus GRK5 enzymatic activity is critical for triggering the nuclear translocation of GRK5 itself (Online Figure V), as well as CaM (Fig 4) and HDAC4/5 nuclear export (Fig 6 and Online Figure VI). We suggest that GRK5 activation is critical, and that it enters the nucleus together with CaM. However, AMX did not entirely block the AngII-induced nuclear CaM accumulation, suggesting either incomplete GRK5 inhibition, that activity may not be an absolute requirement or that some CaM may enter the nucleus by itself via the nuclear pore complex or other pathway. While this working model undoubtedly oversimplifies GRK5’s complex nuclear translocation, CaM and DNA binding, as studied in cultured epithelial cells [32], it provides a working framework for this system in adult cardiac myocytes.
In addition to the acute CaM and GRK5 translocation induced by AngII, PE and suramin in adult ventricular myocyte, we also tested whether this working model could be detected in physiologically chronic pressure overload induced hypertrophy and HF over 2–8 weeks (Fig 7). Indeed, we found that there was an analogous shift in CaM from RyR2 to the nucleus that was accompanied by GRK5 nuclear accumulation, as seen upon acute hypertrophic agonists AngII and PE. This was seen already at the hypertrophic stage (2 weeks post-TAC), and at least for CaM even more pronounced at the HF stage (8 week post-TAC). The failure of GRK5 nuclear accumulation to further increase at 8 weeks could reflect a maximal activation that occurred already at 2 weeks, or that nuclear GRK5 is more important in the hypertrophic phase than the transition to HF, but further elucidation of this would require additional study.
4.3. HDAC nuclear export is triggered by GRK5 and CaM import
Class IIa HDACs (e.g. HDAC4 and 5) regulate cardiac hypertrophic gene transcription [29]. HDAC4 and 5 are phosphorylated by CaMKII, PKD and GRK5, which promotes their nuclear export and derepresses pathological hypertrophic cardiac gene expression driven by MEF2 (Fig 8)[17,21–23,34,35]. Thus, nuclear export of HDAC4 and 5 are key determinants of hypertrophic transcriptional activation. Here, we show that AngII or PE promotes HDAC4/5 nuclear export, that this is mimicked by simply releasing RyR2-bound CaM (suramin), and prevented by stabilizing CaM-RyR2 (dantrolene). These findings suggest that CaM from RyR2 is critical for both GRK5 nuclear import and downstream HDAC nuclear export. It is also consistent with observations in a mouse in which RyR2 CaM binding was suppressed by a genetic RyR2 mutation, where HDAC4 phosphorylation was enhanced (by a kinase other than CaMKII) which lead to cardiac hypertrophy and early death [12]. CaM also binds to and activates the phosphatase calcineurin, which dephosphorylates nuclear factor of activated T cells (NFAT), causing NFAT nuclear import and activation of hypertrophic gene expression [1,36]. Although not tested directly here, CaM released from RyR2 and imported into the nucleus would also be expected to enhance hypertrophic gene expression in this CaM-calcineurin pathway by promoting NFAT nuclear import and retention.
The TAC-induced progressive decline in nuclear/cytosolic HDAC4/5 (from 2 to 8 weeks post-TAC; Fig 7 and Online Figure VI) parallels nuclear CaM accumulation better than it does that of GRK5. This may mean that some other CaM-dependent pathway (e.g. CaMKII activation) becomes more important in the transition from hypertrophy to HF, as seen in CaMKIIδ knockout mice [37].
5. Limitations
There are a several limitations to the study that merit comment here. We relied entirely on immunofluorescence assay for quantitative protein localization. Western blot analysis would sacrifice the spatiotemporal resolution which we used here, and minor changes in CaM affinity that result in spatial shifts would be difficult to quantify via western blots. Having said that, there is strong direct evidence from multiple methods and studies to support the inferred CaM-RyR, and CaM-GRK5 binding as well as the effects of suramin and dantrolene on CaM-RyR binding. Moreover, while Yamaguchi et al. [12] showed that knock-in mice expressing RyR2 unable to bind CaM developed cardiac hypertrophy (consistent with our idea), we have not directly proven here whether RyR2 CaM release by itself would suffice to drive pathological cardiac hypertrophy. Further tests of the working hypothesis that we have developed in Fig 8 using complementary methods and additional animal models would be valuable.
Since dissociation of CaM from RyR2 increases Ca2+ leak via RyR2, it is tempting to speculate that the abnormal SR Ca2+ leak itself promotes pathological cardiac hypertrophy [38]. Moreover, Ljubojevic et al. [39] proposed that structural and functional changes in the nuclear envelope and perinuclear regions alter the nucleoplasmic Ca2+ handling (increasing diastolic [Ca2+]nuc), independently from [Ca2+]cyto, leading to development of cardiac hypertrophy. Future studies will be required to clarify the detailed relation between nucleoplasmic Ca2+ handling and RyR2-CaM movement during cardiac remodeling.
6. Conclusion
Pathological cardiac hypertrophy and HF is a complex process involving multiple signaling pathways. In this study, we show that CaM released from RyR2 is an important trigger that promotes GRK5 nuclear accumulation, and drives the pathological cardiac hypertrophy. In particular, known players that promote the neurohumoral storm in HF (AngII and α-adrenergic receptor activation) activate this RyR2-CaM dissociation and nuclear translocation with GRK5, in addition to increasing RyR2-mediated arrhythmogenic SR Ca2+ release. Normalization of CaM binding affinity to RyR2 and its regulation may, therefore, be a novel therapeutic approach for preventing multiple adverse pathological cardiac pathways.
Supplementary Material
Supplementary Material
Highlights.
Calmodulin (CaM), which dissociated from RyR2, translocated to the
nucleus.
Movement of RyR2-CaM to the nucleus along with GRK5 promotes
pathological cardiac hypertrophy.
Dantrolene prevents RyR2-CaM movement and HDAC5 nuclear export.
Amlexanox, a pharmacological inhibitor of GRK5 kinase activity,
suppressed HDAC5 nuclear export.
Pressure overload causes CaM movement from RyR2 to nucleus with
GRK5 and promotes the nuclear export of class IIa HDAC.
Sources of Funding
This work was supported by JSPS KAKENHI Grant Number 16K09502 (TO), by JSPS KAKENHI Grant (MY) and National Institutes of Health R01-HL092097 (DB).
Non-standard Abbreviations and Acronyms
- AngII
angiotensin II
- AMX
amlexanox
- CaM
calmodulin
- CaMKII
Ca2+/CaM-dependent protein kinase II
- Ctl
control
- CPVT
catecholaminergic polymorphic ventricular tachycardia
- DAN
dantrolene
- DAPI
4’, 6-diamidino-2-phenylindole
- ECC
excitation-contraction coupling
- F
fluorescent
- GRK5
G protein-coupled receptors kinase 5
- HDAC5
histone deacetylase 5
- HDAC4
histone deacetylase 4
- HF
heart failure
- KI
knock-in
- PE
phenylephrine
- ROS
reactive oxygen species
- RyR2
cardiac ryanodine receptor
- SR
sarcoplasmic reticulum
- TAC
transverse aortic constriction
- WT
wild-type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors report no conflicts of interest.
References
- [1].Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signaling pathways. Nat Rev Mol Cell Biol. 2006;7:589–600. [DOI] [PubMed] [Google Scholar]
- [2].Yamaguchi N, Xu L, Pasek DA, Evans KE, Meissner G. Molecular basis of calmodulin binding to cardiac muscle Ca2+ release channel (ryanodine receptor). J Bio Chem. 2003;278:23480–23486. [DOI] [PubMed] [Google Scholar]
- [3].Fruen BR, Bardy JM, Byrem TM, Strasburg GM, Louis CF. Differential Ca2+ sensitivity of skeletal and cardiac muscle ryanodine receptors in the presence of calmodulin. Am J Physiol. 2000;279:C724–C733. [DOI] [PubMed] [Google Scholar]
- [4].Balshaw DM, Yamaguchi N, Meissner G. Modulation of intracellular calcium-release channels by calmodulin. J Mem Biol. 2002;185:1–8. [DOI] [PubMed] [Google Scholar]
- [5].Yang Y, Guo T, Oda T, Chakraborty A, Chen L, Uchinoumi H, Knowlton AA, Fruen BR, Cornea RL, Meissner G and Bers DM. Cardiac myocyte Z-line calmodulin is mainly RyR2-bound, and reduction is arrhythmogenic and occurs in heart failure. Circ Res. 2014;114:295–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ono M, Yano M, Hino A, Suetomi T, Xu XJ, Susa T, Uchinoumi H, Tateishi H, Oda T, Okuda S, Doi M, Kobayashi S, Ikemoto N, Matsuzaki M. Dissociation of calmodulin from cardiac ryanodine receptor causes aberrant Ca2+ release in heart failure. Cardiovasc Res. 2010;87:609–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Uchinoumi H, Yang Y, Oda T, Li N, Alsina KM, Puglisi JL, Chen-Izu Y, Cornea RL, Wehrens XHT, Bers DM. CaMKII-dependent phosphorylation of RyR2 promotes targetable pathological RyR2 conformational shift. J Mol Cell Cardiol. 2016;98:62–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Xu XJ, Yano M, Uchinoumi H, Hino A, Suetomi T, Ono M, Tateishi H, Oda T, Okuda S, Doi M, Kobayashi S, Yamamoto T, Ikeda Y, Ikemoto N, Matsuzaki M. Defective calmodulin binding to the cardiac ryanodine receptor plays a key role in CPVT-associated channel dysfunction. Biochem Biophys Res Commun. 2010; 394:660–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Uchinoumi H, Yano M, Suetomi T, Ono M, Xu X, Tateishi H, Oda T, Okuda S, Doi M, Kobayashi S, Yamamoto T, Ikeda Y, Ohkusa T, Ikemoto N, Matsuzaki M. Catecholaminergic polymorphic ventricular tachycardia is caused by mutation-linked defective conformational regulation of the ryanodine receptor. Circ Res. 2010;106:1413–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Oda T, Yang Y, Uchinoumi H, Thomas DD, Chen-Izu Y, Kato T, Yamamoto T, Yano M, Cornea RL and Bers DM. Oxidation of Ryanodine Receptor (RyR) and Calmodulin enhance Ca release and pathologically alter, RyR structure and Calmodulin affinity. J Mol Cell Cardiol. 2015;85:240–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kato T, Yamamoto T, Nakamura Y, Nanno T, Fukui G, Sufu Y, Hamada Y, Maeda T, Nishimura S, Ishiguchi H, Murakami W, Fukuda M, Xu X, Hino A, Ono M, Oda T, Okuda S, Kobayashi S, Koseki N, Kyushiki H, Yano M. Correction of impaired calmodulin binding to RyR2 as a novel therapy for lethal arrhythmia in the pressure-overloaded heart failure. Heart Rhythm. 2017;14:120–127. [DOI] [PubMed] [Google Scholar]
- [12].Yamaguchi N, Takahashi N, Xu L, Smithies O, Meissner G. Early cardiac hypertrophy in mice with impaired calmodulin regulation of cardiac muscle Ca release channel. J Clin Invest. 2017;117:1344–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ai X, Curran JW, Shannon TR, Bers DM, Pogwizd SM. Ca2+/calmodulin-dependent protein kinase modulates cardiac ryanodine receptor phosphorylation and sarcoplasmic reticulum Ca2+ leak in heart failure. Circ Res. 2005;97:1314–1322. [DOI] [PubMed] [Google Scholar]
- [14].Deisseroth K, Heist EK, Tsien RW. Translocation of calmodulin to the nucleus supports CREB phosphorylation in hippocampal neurons. Nature. 1998;392:198–202. [DOI] [PubMed] [Google Scholar]
- [15].Thorogate R, Torok K. Ca2+-dependent and ‒independent mechanisms of calmodulin nuclear translocation. J Cell Sci. 2004;117:5923–5936. [DOI] [PubMed] [Google Scholar]
- [16].Wu X and Bers DM. Free and bound intracellular calmodulin measurements in cardiac myocytes. Cell Calcium. 2007;41:353–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Martini JS, Raake P, Vinge LE, DeGeorge BR, Chuprun JK, Harris DM, Gao E, Eckhart AD, Pitcher JA, Koch WJ. Uncovering G protein-coupled receptor kinase-5 as a histone deacetylase kinase in the nucleus of cardiomyocytes. Pros Natl Acad Sci USA. 2008;105:12457–12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Pronin AN, Satpaev DK, Slepak VZ, Benovic JL. Regulation of G protein-coupled receptor kinases by calmodulin and localization of the calmodulin binding domain. J Biol Chem. 1997;272:18273–18280. [DOI] [PubMed] [Google Scholar]
- [19].Gold JI, Martini JS, Hullmann J, Gao E, Chuprun JK, Lee L, Tilley DG, Rabinowitz JE, Bossuyt J, Bers DM, Koch WJ. Nuclear translocation of cardiac G protein-coupled receptor kinase 5 downstream of select Gq-activating hypertrophic ligands is a calmodulin-dependent process. PLoS One. 2013;8:e57324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Olson EN, Schneider MD. Sizing up the heart: development redux in disease. Genes Dev. 2003;17:1937–1956. [DOI] [PubMed] [Google Scholar]
- [21].Backs J, Song K, Bezprozvannaya S, Chang S, Olson EN. CaM kinase II selectively signals to histone deacetylase 4 during cardiomyocyte hypertrophy. J Clin. Invest 2006;116:1853–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wu X, Zhang T, Bossuyt J, Li X, McKinsey T, Dedman JR, Olson EN, Chen J, Brown JH, Bers DM. Local InsP3-dependent Perinuclear Ca Signaling in Cardiac Myocytes Excitation-Transcription Coupling. J Clin Invest. 2006;116:675–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Bossuyt J, Chang CW, Helmstadter K, Kunkel MT, Newton AC, Campbell KS, Martin JL, Bossuyt S, Robia S, Bers DM. Spatiotemporally distinct PKD activation in adult cardiomyocytes in response to phenylephrine and endothelin. J Biol Chem. 2011;286:33390–33400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Guo T, Fruen BR, Nitu FR, Nguyen TD, Yang Y, Cornea RL, Bers DM. FRET detection of calmodulin binding to the cardiac RyR2 calcium release channel. Biophys J. 2011;101:2170–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Homan KT, Wu E, Cannavo A, Koch WJ, Tesmer JJG. Identification and Characterization of Amlexanox as a G Protein-Coupled Receptor Kinase 5 Inhibitor. Molecules. 2014;19:16937–16949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Maier LM, Bers DM. Calcium, calmodulin, and calcium/calmodulin kinase II – From heartbeat to heartbeat. J Mol Cell Cardiol. 2002;34:919–939. [DOI] [PubMed] [Google Scholar]
- [27].Anderson ME, Brown JH, Bers DM. CaMKII in Myocardial Hypertrophy and Heart Failure. J Mol Cell Cardiol. 2011;51:468–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Goonasekera SA, Molkentin JD. Unraveling the secrets of a double life: contractile versus signaling Ca2+ in a cardiac myocyte. J Mol Cell Cardiol. 2012;52:317–22. [DOI] [PubMed] [Google Scholar]
- [29].Lifton RP, Gharavi AG, Geller DS. Molecular mechanisms of human hypertension. Cell. 2001;104:545–556. [DOI] [PubMed] [Google Scholar]
- [30].Gardin JM, Lauer MS. Left ventricular hypertrophy: the next treatable, silent killer? JAMA. 2004;292:2396–2398. [DOI] [PubMed] [Google Scholar]
- [31].Gangopadhyay JP, Ikemoto N. Intracellular translocation of calmodulin and Ca2+/calmodulin-dependent protein kinase II during the development of hypertrophy in neonatal cardiomyocytes. Biochem Biophys Res Commum. 2010;396:515–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Johnson LR, Scott MG, Pitcher JA. G protein-coupled receptor kinase 5 contains a DNA-binding nuclear localization sequence. Mol Cell Biol. 2004;24:10169–10179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Chuang TT, Paolucci L, de Blasi A. Inhibition of G protein-coupled receptor kinase subtypes by Ca2+/calmodulin. J Biol Chem. 1996;271:28691–28696. [DOI] [PubMed] [Google Scholar]
- [34].Lu J, McKinsey TA, Zhang CL, Richardson JA, Hill JA, Olson EN. Signal-dependent activation of the MEF2 transcription factor by dissociation from histone deacetylases. Proc Natl Acad Sci USA. 2000;97:4070–4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hohl M, Wagner M, Reil JC, Muller SA, Tauchnitz M, Zimmer AM, Lehmann LH, Thiel G, Bohm M, Backs J, Maack C. HDAC4 controls histone methylation in response to elevated cardiac load. J Clin Invest.2013;123:1359–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Grabtree GR, Olson EN. NFAT signaling: choreographing the social lives of cells. Cell. 2002;109:S67–S79. [DOI] [PubMed] [Google Scholar]
- [37].Ling H, Zhang T, Pereira L, Means CK, Cheng H, Gu Y, Dalton ND, Peterson KL, Chen J, Bers D, Brown JH. Requirement for Ca2+/calmodulin-dependent kinase II in the transition from pressure overload-induced cardiac hypertrophy to heart failure in mice. J Clin Invest. 2009;119:1230–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Sedej S, Schmidt A, Denegri M, Walther S, Matovina M, Arnstein G, Gutschi EM, Windhager I, Ljubojevic S, Negri S, Heinzel FR, Bisping E, Vos MA, Napolitano C, Priori SG, Kockskamper J, Pieske B. Subclinical abnormalities in sarcoplasmic reticulum Ca release promote eccentric myocardial remodeling and pump failure death in response to pressure overload. J Am Coll Cardiol. 2013;63:1569–79. [DOI] [PubMed] [Google Scholar]
- [39].Ljubojevic S, Radulovic S, Leitinger G, Sedej S, Sacherer M, Holzer M, Winkler C, Pritz E, Mittler T, Schmidt A, Sereinigg M, Wakula P, Zissimopoulos S, Bisping E, Post H, Marsche G, Bossuyt J, Bers DM, Kockskamper J, Pieske B. Early remodeling of perinuclear Ca2+ stores and nucleoplasmic Ca2+ signaling during the development of hypertrophy and heart failure. Circulation. 2014;130:244–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.