Abstract.
Maternal infection during pregnancy can have lasting effects on neurodevelopment, but the impact of malaria in pregnancy on child neurodevelopment is unknown. We present a case of a 24-year-old gravida three woman enrolled at 14 weeks 6 days of gestation in a clinical trial evaluating malaria prevention strategies in pregnancy. She had two blood samples test positive for Plasmodium falciparum using loop-mediated isothermal amplification before 20 weeks of gestation. At 31 weeks 4 days of gestation, the woman presented with preterm premature rupture of membranes, and the twins were delivered by cesarean section. Twin A was 1,920 g and Twin B was 1,320 g. Both placentas tested negative for malaria by microscopy, but the placenta of Twin B had evidence of past malaria by histology. The twins’ development was assessed using the Bayley Scales of Infant and Toddler Development–Third Edition. At 1 year chronologic age, Twin B had lower scores across all domains (composite scores: cognitive, Twin A [100], Twin B [70]; motor, Twin A [88], Twin B [73]; language, Twin A [109], Twin B [86]). This effect persisted at 2 years chronologic age (composite scores: cognitive, Twin A [80], Twin B [60]; motor, Twin A [76], Twin B [67]; language, Twin A [77], Twin B [59]). Infant health was similar over the first 2 years of life. We report differences in neurodevelopmental outcomes in placental malaria-discordant dizygotic twins. Additional research is needed to evaluate the impact of placental malaria on neurodevelopmental complications. Trial registration number: ClinicalTrials.gov number, NCT02163447. Registered: June 2014, https://clinicaltrials.gov/ct2/show/NCT02163447.
INTRODUCTION
There are emerging data showing that maternal infection during pregnancy can have lasting effects on child neurodevelopment.1 Present evidence suggests that in utero exposure to maternal immune activation can disrupt early neurodevelopmental processes resulting in subsequent neuropsychiatric and neurological disorders.2 Although most pregnancies in sub-Saharan Africa are at risk of malaria in pregnancy (MIP),3 the impact of MIP on child neurodevelopment is unknown. Preclinical data from an experimental model of MIP show that in utero exposure to MIP is associated with persistent neurocognitive deficits in memory and affective-like behavior compared with unexposed controls, independent of a low birth weight phenotype.4 However, this has not been evaluated in clinical studies. Here, we describe neurodevelopmental outcomes associated with a set of dizygotic placental malaria-discordant twins.
CASE REPORT
Between June and October 2014, HIV-uninfected pregnant women were enrolled in a double-blind, randomized, controlled trial evaluating the safety and efficacy of intermittent preventive treatment of malaria during pregnancy.5 This study was conducted in Tororo, Eastern Uganda, where malaria transmission intensity is estimated at 310 infectious bites per person-year,6 and the prevalence of sulfadoxine-pyrimethamine resistance is high. Women were monitored throughout pregnancy for clinical malaria, and blood samples were collected at monthly scheduled visits for molecular assessment of Plasmodium falciparum using loop-mediated isothermal amplification (LAMP).
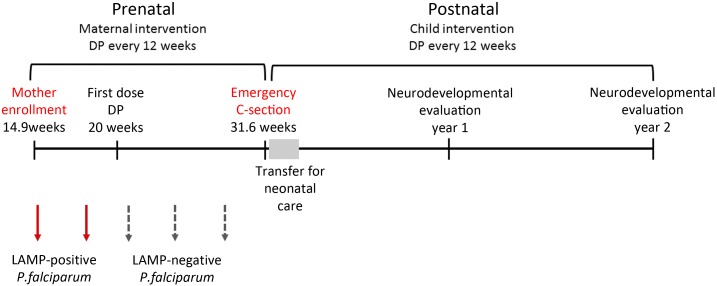
A 24-year-old gravida three woman was enrolled in the study at 14 weeks 6 days of gestation by ultrasound dating. An ultrasound scan revealed a twin pregnancy with no size differences noted between fetuses. The mother had a hemoglobin level of 11.0 g/dL, tested negative for malaria by microscopy, and was randomized to receive three doses of dihydroartemisinin–piperaquine (DP) in pregnancy (three tablets of 40 mg dihyroartemisinin and 320 mg of piperaquine [Duo-Cotecxin, Holley-Cotec] given once a day for three consecutive days). At 20 weeks of gestation, the mother received her first dose of DP. She reported no history of hypertension or diabetes mellitus, and did not drink alcohol or smoke tobacco during pregnancy. Routine malaria testing using LAMP revealed that the mother had been positive for malaria at enrollment (14.9 weeks of gestation) and in the immediate follow-up sample (16–19 weeks of gestation). All subsequent tests were negative (Figure 1).
Figure 1.
Schedule of study follow-up. Diagram depicting documented malaria exposure during pregnancy and maternal and child interventions over study period. The infants were delivered at 31.6 weeks of gestation and transferred to Mbale hospital for neonatal care for a week before discharge. At 1 and 2 year’s postnatal ages the twins were evaluated using the Bayley’s Scales of Infant and Toddler Development–Third Edition. This figure appears in color at www.ajtmh.org.
At 31 weeks 4 days, the woman returned to the study clinic with preterm premature rupture of membranes (PPROM) with drainage of clear liquid and complaints of lower abdominal pains. She had no complaints of fever or other associated symptoms. Her cervix was thin and soft and she was 6 cm dilated. On examination, the first twin was breech and the second transverse. Considering the prematurity and the first twin presenting breech, an emergency cesarean section was carried out. There were no reported complications during the cesarean section. The Apgar scores were nine at 1 minute and 10 at 5 minutes following birth for both twins. There were no congenital abnormalities or dysmorphic features detected and both infants had normal reflexes and muscle tone. Twin A was born 1,920 g with a head circumference of 32 cm, and a length of 43 cm, and Twin B was born 1,320 g with a head circumference of 27 cm, and a length of 38 cm. Although the size discrepancies between twins were notable with a 31.3% birth weight discordance, the head circumference for both twins was in the normal range (Twin A, 98.0%; Twin B, 12.3%) and neither was small-for-gestational age (< 10th percentile for gestational age) according to INTERGROWTH 21st standards.7 The twins were dichorionic suggesting that twin-to-twin transfusion syndrome did not contribute to the growth discordance.8
At delivery, cord blood and placental blood were negative for malaria parasites by microscopy and LAMP. Placental blood was collected by making a small incision on the maternal surface of the placenta within 1 hour of delivery. Placental tissue was collected for histological assessment as previously described.5 Briefly, two 1-cm-wide full-thickness biopsies were collected from each placenta, placed in 10% neutral buffered formalin, embedded in paraffin wax, sectioned into 3 uM slices, fixed to glass slides, and stained in 0.1% hematoxylin and 1% eosin for 5 and 1 minute respectively, or in 2% Giemsa for 30 minutes. For quantification, 1,000 intervillous blood cells were counted under high power. Percentages of intervillous erythrocytes infected with parasites and of monocyte/macrophages containing malarial pigment were counted. Placental specimens were examined by three readers. No parasites were detected in either placenta by histology, but hemozoin was detected in the placenta of Twin B, indicating past placental malaria infection. Placental pathology showed no evidence of intervillous inflammation in either twin. Chorioamnionitis was assessed by an expert pathologist and there was no evidence of chorioamnionitis for either twin. Placental weights were not recorded.
Considering the prematurity of the infants, they were transferred to the Mbale Regional Referral Hospital neonatal ward where they received routine neonatal care including phototherapy for jaundice. There is no medical documentation of their neonatal care, but the mother did not perceive any differences in medical care between the twins. The twins were hospitalized for the same period of time with a similar duration of phototherapy, similar feeding profiles, and no differential respiratory distress or apnea reported. According to the mother, there were no fevers or complications during hospitalization or the neonatal period, and the infants were exclusively fed breastmilk with no formula supplementation, although the larger twin fed more. Additional details related to history of hypoglycemia, kernicterus, or respiratory distress/hypoxia in these high-risk infants are not available.
The children were randomized to receive DP every 12 weeks following birth and were followed-up until the age of 2 years. At the 1 year follow-up visit, the mother reported delays in sitting/standing/walking, difficulties in learning, and differences in speech for both twins using the 10 Questions Questionnaire.9 These findings may be related to the prematurity of the twins as they had a corrected postnatal age of 46.1 weeks (10.6 months) at the time of evaluation (scheduled for 12 months chronologic age). The Bayley Scales of Infant and Toddler Development–Third Edition,10 was used for evaluation, with Twin B having lower composite scores across all domains (Table 1). A decline in two standard deviations (30 points in overall cognition) was observed between twins suggesting that the difference is both clinically and statistically significant. Infant health was similar over the first year of life. Both twins had anemia diagnosed at a scheduled follow-up visit (hb < 10 g/dL) and had an out-patient sick visit for pneumonia. In addition, Twin B had a sick visit for a 3-day history of fever and a tender fluctuant swelling on the knee that was drained, and the child was administered paracetamol and antibiotics. Neither twin had malaria, an acute diarrheal illness, or was hospitalized.
Table 1.
Bayley composite scores over the first 2 years of life
| Year 1 | Year 2 | |||
|---|---|---|---|---|
| Twin A | Twin B | Twin A | Twin B | |
| Cognitive | 100 | 70 | 80 | 60 |
| Motor | 88 | 73 | 76 | 76 |
| Language | 109 | 86 | 77 | 59 |
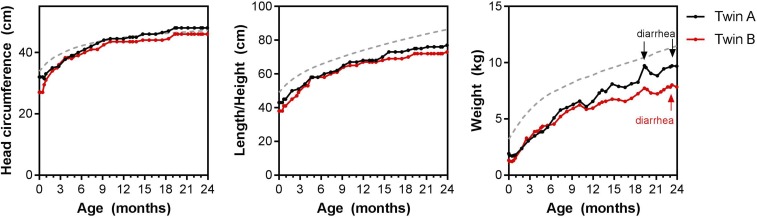
At the scheduled neurocognitive follow-up appointment at 2 years chronologic age, the developmental difference persisted with Twin B showing consistent delays across domains (Table 1). The difference in overall cognition was reduced to 20 points (greater than one standard deviation). Twin A had a total of six sick visits over year 2, with two visits for upper respiratory tract infections, two visits for an acute diarrheal episode, and was diagnosed with a fungal skin infection and pustular skin infection. Twin B had a total of nine sick visits over year 2 with an upper respiratory tract infection, sepsis, common cold, an acute diarrheal episode, fungal skin infection, pustular skin infection, a wound, bacterial skin infection, and gastritis. Neither twin had malaria or required hospitalization. Comparison of the twins’ growth over the age of 2 years revealed that Twin B remained smaller than Twin A, with the differences most evident in the second year of life (Figure 2).
Figure 2.
Growth trajectory over the first 2 years of life. Head circumference, length/height, and weight taken at all scheduled and unscheduled visits (months calculated as number of days from birth/30.42). The dotted gray reference line is the 50 percentile based on the World Health Organization growth chart for girls. This figure appears in color at www.ajtmh.org.
DISCUSSION
This case report has limitations. The twins were born premature in a low-resource setting where complications associated with prematurity and low birth weight may have gone unrecognized because of limitations in clinical and laboratory monitoring. Furthermore, most of these patients’ histories were obtained from the mother’s recall. The cause of the PPROM and labor onset are unknown, but is likely unrelated to the asymptomatic submicroscopic malaria infection that occurred before 20 weeks of gestation.
Histological examination of the placentas revealed evidence of past infection in one placenta, but not the other. Placental histopathology is considered the reference standard for diagnosis of placental malaria, but the sensitivity of histology can be impacted by the quality and number of sections taken. Although the placentas were collected and processed according to established protocols, we cannot rule out infection in the other placenta that was missed as a result of unequal distribution of malaria in the placenta.
As the twins were dizygotic, genetic factors may have contributed to differences in fetal size, placental susceptibility to malaria, and neurodevelopment. Furthermore, because Twin B was born with a very-low birth weight (< 1,500 g), she was at a higher risk for complications that could have contributed to differences in neurodevelopment. An evaluation of 119 growth-discordant twin pairs in the NOTES study, who were evaluated between 24 and 42 months of age using the Bayley Scales and Infant and Toddler Development–Third Edition, found a consistent neurodevelopmental disadvantage of the smaller twin compared with the larger twin, with mean differences (95% CI) in composite scores in cognition, language, and motor development of −1.7 (−3.1, −0.3), −1.8 (−3.3, −0.3), and −2.2 (−4.0, −0.4), respectively.11 Another study of developmental outcomes at an age of 3 years in growth-discordant, very low birth weight, premature twins noted the largest developmental difference in twins with a growth discordance greater than 30%.12 Given the level of growth discordance in the twins described in this report, it is not unexpected that the smaller twin had lower composite scores in cognition, language, and motor development; however, the degree of discordance in neurodevelopment described in this set of twins is considerably larger than would be expected on the basis of growth discordance alone, suggesting there are other factors involved in the developmental differences observed.
This case report highlights placental malaria discordance in a set of twins in a scenario of documented submicroscopic infection in the mother where the malaria-exposed infant had worse birth and neurodevelopmental outcomes. Given the resource constraints in our setting, we cannot comment on numerous complications that may have gone unrecognized. Certainly other prenatal factors (e.g., placental ischemia or inflammation) or postnatal factors (e.g. discrepancy in risks for poor outcomes in low birth weight and very low birth weight infants including intraventricular hemorrhage, hypoglycemia, hypothermia, respiratory distress, apnea, among others) could explain the differential neurodevelopmental outcomes. However, in the absence of clear medical documentation of differential clinical courses and with clear histopathologic differences in the placentas for the twins, this case raises interesting questions about the possibility for long-term neurodevelopmental effects arising from in utero infection. This report highlights a lack of knowledge regarding factors that contribute to placental malaria susceptibility, and supports the need for studies to evaluate the impact of malaria exposure in pregnancy on neurodevelopment.
Acknowledgments:
We would like to thank the mother and her children for participating in the study, the dedicated research team for providing excellent clinical care and collecting data, and the team of neurocognitive testers for their efforts.
REFERENCES
- 1.Bilbo SD, Schwarz JM, 2009. Early-life programming of later-life brain and behavior: a critical role for the immune system. Front Behav Neurosci 3: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knuesel I, Chicha L, Britschgi M, Schobel SA, Bodmer M, Hellings JA, Toovey S, Prinssen EP, 2014. Maternal immune activation and abnormal brain development across CNS disorders. Nat Rev Neurol 10: 643. [DOI] [PubMed] [Google Scholar]
- 3.Dellicour S, Tatem AJ, Guerra CA, Snow RW, Kuile FO, 2010. Quantifying the number of pregnancies at risk of malaria in 2007: a demographic study. PLoS Med 7: e1000221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McDonald CR, et al. 2015. Experimental malaria in pregnancy induces neurocognitive injury in uninfected offspring via a C5a-C5a receptor dependent pathway. PLoS Pathog 11: e1005140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kakuru A, Jagannathan P, Muhindo MK, Natureeba P, Awori P, Nakalembe M, 2016. Dihydroartemisinin–piperaquine for the prevention of malaria in pregnancy. N Engl J Med 374: 928–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kamya MR, et al. 2015. Malaria transmission, infection, and disease at three sites with varied transmission intensity in Uganda: implications for malaria control. Am J Trop Med Hyg 92: 903–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Villar J, et al. International Fetal and Newborn Growth Consortium for the 21st Century (INTERGROWTH-21st) , 2014. International standards for newborn weight, length, and head circumference by gestational age and sex: the newborn cross-sectional study of the INTERGROWTH-21st project. Lancet 384: 857–868. [DOI] [PubMed] [Google Scholar]
- 8.Society for Maternal-Fetal Medicine Simpson LL, 2013. Twin-twin transfusion syndrome. Am J Obstet Gynecol 208: 3–18. [DOI] [PubMed] [Google Scholar]
- 9.Zaman SS, Khan NZ, Islam S, Banu S, Dixit S, Shrout P, Durkin M, 1990. Validity of the ‘ten questions’ for screening serious childhood disability: results from urban Bangladesh. Int J Epidemiol 19: 613–620. [DOI] [PubMed] [Google Scholar]
- 10.Bayley N, 2006. Bayley Scales of Infant and Toddler Development, 3rd edition San Antonio, TX. [Google Scholar]
- 11.Halling C, Malone FD, Breathnach FM, Stewart MC, McAuliffe FM, Morrison JJ, Dicker P, Manning F, Corcoran JD, Perinatal Ireland Research Consortium , 2016. Neuro-developmental outcome of a large cohort of growth discordant twins. Eur J Pediatr 175: 381–389. [DOI] [PubMed] [Google Scholar]
- 12.Goyen T-A, Veddovi M, Lui K, 2003. Developmental outcome of discordant premature twins at 3 years. Early Hum Dev 73: 27–37. [DOI] [PubMed] [Google Scholar]