Abstract.
Enteric pathogens can be transmitted within the household and the surrounding neighborhood. The objective of this study was to understand the effect of neighborhood-level sanitation coverage on contamination of the household environment with levels of fecal indicator bacteria in rural Bangladesh. We conducted spot-check observations of sanitation facilities in neighboring households (NHs) within a 20-m radius of target households with children aged 6–24 months. Sanitation facilities were defined as improved (a private pit latrine with a slab or better) or unimproved. Fecal coliforms (FCs) on children’s hands and sentinel toy balls were measured and used as indicators of household-level fecal contamination. We visited 1,784 NHs surrounding 428 target households. On average, sentinel toy balls had 2.11(standard deviation [SD] = 1.37) log10 colony-forming units (CFUs) of FCs/toy ball and children’s hands had 2.23 (SD = 1.15) log10 CFU of FCs/two hands. Access to 100% private improved sanitation coverage in the neighborhood was associated with a small and statistically insignificant difference in contamination of sentinel toy balls (difference in means = −0.13 log10 CFU/toy ball; 95% confidence intervals [CI]: −0.64, 0.39; P = 0.63) and children’s hands (difference in means = −0.11 log10 CFU/two hands; 95% CI: −0.53, 0.32; P = 0.62). Improved sanitation coverage in the neighborhood had limited measurable effect on FCs in the target household environment. Other factors such as access to improved sanitation in the household, absence of cow dung, presence of appropriate water drainage, and optimal handwashing practice may be more important in reducing FCs in the household environment.
INTRODUCTION
Enteric pathogens excreted in feces can be transmitted through contaminated food and drink, from person to person (hand to mouth), or from contact with a fomite or flies either through contaminated food and utensils or landing directly on children.1–4 In rural areas of densely populated countries, households live very close to each other. Members of neighboring households (NHs) often share a yard along with basic water and sanitation infrastructure.5 This allows frequent movement of adults and children between households within the neighborhood, resulting in enteric pathogens spread across households and the surrounding community.6–8
Sanitation facilities that separate feces from the environment are expected to create a primary barrier to break the chain of transmission of enteric pathogens.1,2 Sanitation may prevent transmission of enteric pathogens in two ways. There may be a direct benefit to a household due to improving household sanitation. There may be also an additional external benefit due to immediate neighbor’s access to sanitation that result in a lower probability of human contact with human excreta.9 We have limited empirical evidence to understand whether the benefits of sanitation at household level critically depend on sanitation coverage across the neighborhood.10
Several studies have assessed the effect of community sanitation coverage on health. A few studies have looked at the effect of community coverage of sanitation facilities connected to sewer systems or septic tanks in urban contexts.11–16 These studies show the importance of community sanitation access, but they do not clarify the role of neighborhood sanitation on target households in preventing fecal contamination and related health outcomes. From these studies, we do not know if sanitation provides an externality benefit due to immediate neighbor’s access to sanitation.
A study conducted in Brazil assessed the effect on child diarrhea of a city-wide intervention to improve sewerage coverage. Following the intervention, there was a 22% reduction in the longitudinal prevalence of diarrhea. Household-level sanitation-related variables (indoor latrine and household excreta disposal) explained only 17% of the heterogeneity of the program effect, whereas neighborhood sanitation coverage through sewerage connection explained 100% of the heterogeneity.14 This suggests that in this setting, neighborhood-level sanitation access was more important than household-level sanitation access in preventing diarrheal disease transmission. In most low-income rural settings, sewage connections are not feasible. As of 2010, 60% of global urban residents reported using facilities linked to sewers compared with only 12% in rural areas.17 Most sanitation facilities in rural areas of low-income countries are onsite (pit latrines, septic tanks, and other household-level technologies that do not involve sewerage). In 2010, 64% of the global rural population reported using onsite sanitation facilities.17 In rural settings with predominantly onsite sanitation, the impact of neighborhood sanitation may be different.
Studies conducted in rural contexts with predominantly onsite sanitation facilities have also highlighted that neighborhood sanitation coverage may be important.10,18 However, from these studies, we cannot understand the benefits of externalities due to sanitation access in the neighborhood.
Other studies conducted in the rural context suggest that neighborhood sanitation may provide additional externality benefits in terms of preventing diarrhea.9 For example, a study used data from an Indian nationwide survey of rural households. The findings suggest that community-level improved sanitation coverage is associated with a 37% additional reduction in diarrhea prevalence, in addition to reduction due to household-level improved sanitation coverage.9 A second study that used Demographic and Health Survey (DHS) data suggests that children from villages with a higher open defecation rate were shorter after controlling for effect of household-level sanitation practices.19 Similar studies have so far not been repeated in other settings. Depending on prevalence of a disease in a specific context, the effect of risk factors such as lack of sanitation may have variable effect in reducing the disease.20,21 For example, in a given context, improving sanitation may reduce diarrheal disease prevalence but in a similar setting when the prevalence is lower, sanitation interventions may no longer reduce prevalence of diarrheal disease. The classification of sanitation facilities in DHSs may be prone to misclassification as the categories used in the DHSs do not capture the function of sanitation facilities in separating feces from environment.
The objective of this study was to assess the association between neighborhood sanitation coverage and contamination of the household environment with levels of fecal indicator bacteria to help inform decisions regarding the focus of sanitation interventions and how we monitor global progress.
METHODS
We conducted an observational, cross-sectional study between September and October 2013, in rural areas of Mymensingh and Narshingdi districts of Bangladesh. We conducted the study in villages that were participating in the Sanitation, Hygiene Education, and Water supply in Bangladesh health impact study described elsewhere.22 We conducted verbally administered questionnaire surveys, spot-check of sanitation facilities, and microbial assessment of children’s hands and sentinel toy ball (described in the following paragraphs).
Neighboring household selection.
The enumerators systematically selected 454 target households with a child aged 6–24 months, from a simple random sample of villages enrolled for a health impact study as described elsewhere.23 The youngest child aged 6–24 months of age in the target household was considered as the target child. All NHs within a 20-m radius of the entrance to the living room of each target household were enrolled in this study. We define the term “neighborhood” as the immediate NHs. The distance between households was measured using a handheld global positioning system unit “Garmin Etrex legend H” (GARMIN, Southampton, Hampshire, UK).24 Target households were separated by a distance of at least 50 m ensuring that none of the NHs was counted for more than one target household.
Data collection tools.
Neighborhood and target household data were collected by enumerators that used a verbally administered, structured questionnaire and spot-check observations on household possessions; water, sanitation, and hygiene-related behavior and facilities in target households.23 Enumerators used a shorter version of this procedure to collect information about human and animal feces disposal practices in the NHs. Data were recorded using a tablet computer.
Microbiological sample collection.
We used contamination of toys and hands by fecal indicator bacteria as an indicator of fecal contamination of the household environment. Throughout the article, the term “fecal contamination” refers to contamination with fecal indicator bacteria.
Hand rinse: Before administering the household survey, the field team rinsed both hands of the target child (aged 6–24 months) from each target household. Hands were rinsed for 30 seconds each, in a Whirl-Pak bag (19 × 38 cm) (Nasco, Fort Atkinson, WI) filled with 200 mL of Ringer’s solution.25
Sentinel toy ball rinse: Standard-sized (20 cm circumference) sentinel toy balls (Figure 1) given to children to play with were collected after 24 hours and rinsed in a Whirl-Pak bag (19 × 38 cm) filled with 200 mL of Ringer’s solution for 30 seconds following the methods used previously.26
Figure 1.
Sentinel toy ball. This figure appears in color at www.ajtmh.org.
All samples were transported in a cool box to the icddr,b Environmental Microbiology Laboratory within 15–18 hours of collection, maintaining a temperature of 4–10°C.
Enumeration of fecal coliforms (FCs).
Fecal coliforms were enumerated using a membrane filtration technique with modified FC agar plates, within 24 hours of collection.27,28 The results were calculated as colony-forming units (CFUs) present per 200 mL of recovered media that bathed the toy balls or hands.
Ethics.
Written informed consent was taken from the primary caregiver of the child aged 6–24 months before enrolling in the study. The study protocol was approved by the Ethical Review Committee of icddr,b, Bangladesh, and London School of Hygiene and Tropical Medicine, United Kingdom.
Operational definitions of variables used in the analysis.
Our analysis included the following variables: neighborhood sanitation coverage (primary exposure variable), household access to improved sanitation, household wealth, latrine cleanliness, hand cleanliness, appropriate child feces disposal, and FC counts (primary outcome variable) from hands and sentinel toys. These variables are defined in the following paragraph.
Access to improved sanitation: We categorized access to improved sanitation using definition used by the WHO/United Nations Children's Fund (UNICEF) Joint Monitoring Program (JMP) for water supply and sanitation.29 Following the JMP, we categorized flush/pour flush latrines and pit latrines with slabs as improved, provided these were not shared between households. Although during formulation of the indicators for the Sustainable Development Goals it was proposed that if a sanitation facility is shared by maximum five households, it will be considered as improved, the final definition did not use the definition of < 5 versus > 5 households because of lack of evidence that five households were a reliable threshold for classifying one sanitation facility as safe and another unsafe. Unimproved sanitation included pit latrines without slabs, hanging latrines, and flush/pour flush latrines with no connection to a sewer or septic tank, that is, no facility. Shared facilities of improved design are considered as “limited sanitation” by JMP and here they were grouped together with unimproved.
Neighborhood sanitation coverage (primary variable of interest): We calculated neighborhood sanitation coverage as the proportion of NHs with access to improved latrines. We treated the neighborhood improved sanitation coverage variable in two different forms: 1) categorical (no improved sanitation in the NH, 1–25% coverage, 26–50% coverage, and 50–100% coverage) and 2) binary (100% coverage and < 100% coverage).
Household wealth: To assess the wealth of target households, we used principal component analysis with 23 household characteristics, excluding sanitation and water access.30,31 We calculated the means, frequencies, and score coefficients and used the correlation matrix of the 23 variables to calculate sample weights.30,32,33 We initially divided the wealth score into quintiles (lower, lower middle, middle, upper middle, and upper). Then we recoded the wealth score as a binary variable rich (upper wealth quintile) or poor (lower, lower middle, middle, and upper middle wealth quintiles).
Hand cleanliness: If the trained enumerators observed no visible dirt on the hands or under the nails of the target child, then the child was considered to have clean hands.
Appropriate water drainage system: A household was considered to have an appropriate water drainage system if a cemented/non-cemented or soak pit was found for disposal of domestic waste water.
Latrine cleanliness: We considered a household to have a clean latrine if the enumerators observed no feces on the slab/floor and pan of the latrine at the time of visit.
Safe child’s feces disposal: The feces of children (younger than 3 years) were considered to be disposed safely if they were reported to be disposed inside a latrine.34
Data analysis.
We first converted the FC concentrations to their base 10 logarithms for calculating means. A FC level of < 1 was replaced with the value 0.5 (half the detection limit) before the conversion. We calculated the difference in log10-transformed arithmetic mean CFU of FCs comparing households with different levels of sanitation coverage in the neighborhood using a linear regression model. To account for the clustering effect at village level, we used a generalized least squares random effects model explicitly allowing the average outcome to vary between village clusters.35–39
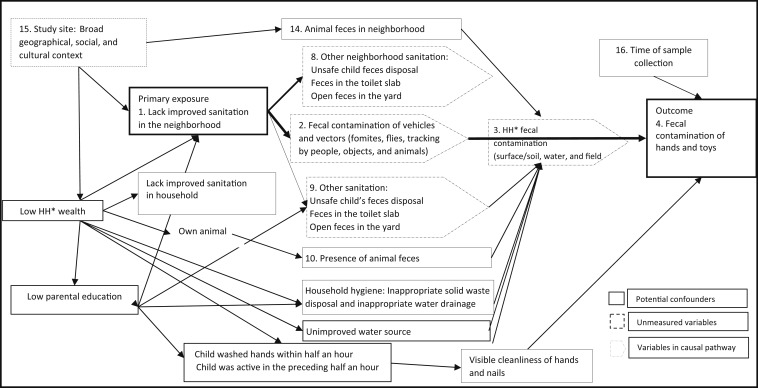
We used causal diagrams to decide which variables to include in the univariable analysis as potential confounders, excluding variables on the same causal pathway as the exposure variables (Figure 2). We then conducted univariable analyses to estimate the crude effect of the primary exposure variable (improved sanitation coverage in the neighborhood) and potential confounding variables on the main outcome, adjusting for the effect of village-level clustering. We decided a priori to include mother’s education and wealth as confounders even if they were not associated with the outcome in the univariable analysis. We included all potential confounders in the multivariable model if they were associated with the exposure and outcome in the univariable analysis.39,40 For a variable to be considered associated with the outcome in the univariable analysis, we used P < 0.1 as the cutoff instead of the conventional P < 0.05 so as to be more inclusive in our effort to incorporate all potential confounders. However, for associations based on adjusted analyses, using multivariable models, we did not consider a specific threshold P-value. Rather, we considered the smaller the P-value, the stronger the evidence rejecting the null hypothesis. We also tested for normality of residuals and homoscedasticity of the models.
Figure 2.
Directed acyclic graph showing the variables measured and included in the multivariable analysis.
We generated separate multivariable models for toy contamination and hand contamination as outcomes. For each of the outcomes, we used two different forms for neighborhood-improved sanitation coverage variable (categorical and binary).
RESULTS
Neighborhood characteristics.
The 454 target households visited had a mean of four NHs within a 20-m radius. Twenty-two target households had no NH within a 20-m radius and an additional four target households had one NH, but none of the family members of those NHs were present during data and sample collection. In this article, we present data from 1,784 NHs around 428 target households. These NHs had five members on average and 35% (n = 684) had at least one child younger than 5 years. The majority (n = 1,431, 80%) of the NHs had animal feces present within the household premises at the time of observation. Among these, 24% (n = 467) had more than 10 piles of open poultry feces and 11% (n = 213) had more than 10 piles of cow dung, whereas 16% (n = 321) had goat feces present (Table 1).
Table 1.
Neighborhood household characteristics
| Household level data (N = 1,784)* | Neighborhood level data† | |||
|---|---|---|---|---|
| n | % or mean | n‡ | Mean (±SD) | |
| Mean number of HH member | 1,784 | 4.6 | 428 | 4.6 (±1.1) |
| Proportion of HH with a < 5-year child | 684 | 35% | 428 | 37% (±29%) |
| Proportion of HH with access to a latrine | 1,672 | 94% | 428 | 93% (19%) |
| Proportion of HH with worn path to latrine§ | 1,666 | 99% | 428 | 93% (±20%) |
| Sanitation access according to technology (ignoring sharing) | ||||
| Open defecation | 102 | 5.7% | 428 | 6% (±19%) |
| Pit latrine without a slab, hanging latrine, and pit latrine with a slab but broken pit | 858 | 48% | 428 | 46% (±37%) |
| Pit latrine with a slab | 436 | 24% | 428 | 25% (±33%) |
| Flush/pour flush latrine with a septic tank or pit | 388 | 22% | 428 | 22% (±32%) |
| Proportion of HH that privately owns a latrine§ | 862 | 51% | 428 | 51% (±34%) |
| Proportion of HH with access to a shared latrine§ | 1,012 | 60% | 428 | 54% (±36%) |
| Mean number of individuals using a latrine | 1,682 | 8 | 428 | 7.6 (±3.2) |
| Mean number of HH sharing a latrine | 1,012 | 2.9 | 428 | 2.7 (±0.9) |
| Sanitation access according to Joint Monitoring Program classification | ||||
| Private improved | 389 | 22% | 428 | 24% (±30%) |
| Unimproved | 1,395 | 78% | 428 | 76% (±30%) |
| Proportion of HH with a dirty latrine§ | 272 | 17% | 428 | 16% (±27%) |
| Reported < 5 child feces at the defecation site | ||||
| Open: Filed/bush/yard/floor | 390 | 57% | 428 | 22% (±27%) |
| Potty | 80 | 12% | 428 | 4% (±12%) |
| Nappy | 69 | 10% | 428 | 4.4% (±14%) |
| In a latrine | 145 | 21% | 428 | 6.9% (±15%) |
| Safe child feces disposal§ | 93 | 22% | 428 | 5% (±14%) |
| Number of piles of poultry feces found in or around HH | ||||
| No feces | 613 | 31% | 428 | 32% (±39%) |
| 1–10 piles | 868 | 45% | 428 | 50% (±40%) |
| 10 > piles | 467 | 24% | 428 | 28% (±37%) |
| Number of piles of cow dung found in or around HH | ||||
| No feces | 1,323 | 68% | 428 | 70% (±43%) |
| 1–10 piles | 412 | 21% | 428 | 25% (±33%) |
| 10 > piles | 213 | 11% | 428 | 14% (±28%) |
| Goat feces found in or around HH | ||||
| Present | 1,627 | 84% | 428 | 19% (±31%) |
| Absent | 321 | 16% | 428 | 90% (±38%) |
HH = household; NHs = neighboring households.
* Here data on all NHs are presented without considering the study design element
† Here, data are presented considering the study design. The study design involved sampling clusters of households (neighborhood), with one “target” HH and n(i) of surrounding HHs, where i is a variable. For this, we first calculated the mean or proportions for the NHs for each target HH and then we presented the mean and standard deviation of the neighborhood characteristics for the target HHs.
‡ Here, “n” represents number of neighborhoods.
§ For these variables, N (denominator) is smaller than 1,784.
Among the NHs, 1,672 (94%) reported having access to a latrine. Almost all (99%) of the households with latrine access had a worn path to the latrine, suggesting regular use. Almost all of these households (99%) reported using the latrine within the 24 hours preceding spot-checks. Among the NHs 60% (n = 1,012), reported access to a shared latrine. About 22% of the households had a flush or pour flush latrine with a septic tank or a pit, whereas 24% households reported to have access to a pit latrine without flush technology. Twenty-two percent of the households had access to a private improved latrine. There were 1,615 households that had a latrine with a slab. Seventeen percent of these latrines were visibly clean (Table 1). Shared latrines were more likely to be dirty than individual latrines (182/969 = 19% versus 90/646 = 14%, P = 0.01). Among 432 households (subset of the 1,784 NHs), with one or more children younger than 3 years, 22% reported that they disposed of the child’s feces in a latrine.
Target household characteristics.
Almost a quarter (24%) of the target households had access to private improved sanitation (Table 2). Only 5% of the households had no access to a toilet. Characteristics of the target households have been presented in more detail elsewhere.23
Table 2.
Relationship between neighborhood (NH) sanitation and log10-transformed fecal coliform colony-forming units/toy ball (N = 428 HHs)
| Exposures | n (%)* | Mean (standard deviation) | Median | Univariable | Multivariable | ||
|---|---|---|---|---|---|---|---|
| Difference in mean† (95% CI) | P value† | Difference in mean†‡ (95% CI)‡ | P-value†‡ | ||||
| Primary exposure: NH sanitation coverage | |||||||
| 1a. Proportion of improved sanitation coverage in the NH‖ | |||||||
| No improved sanitation | 204 (48) | 2.07 (1.45) | 1.90 | – | – | – | – |
| Up to 25% coverage | 80 (19) | 2.08 (1.20) | 2.14 | 0.01 (−0.37, 0.34) | 0.94 | −0.08 (−0.43, 0.27) | 0.67 |
| 26–50% coverage | 88 (21) | 2.30 (1.38) | 2.36 | 0.19 (−0.15, 0.54) | 0.26 | 0.14 (−0.2, 0.48) | 0.42 |
| 51–100% coverage | 56 (13) | 2.04 (1.26) | 1.90 | −0.07 (−0.48, 0.33) | 0.72 | 0.04 (−0.38, 0.46) | 0.84 |
| 1b. Improved sanitation coverage in the NH‖ | |||||||
| 100% coverage | 29 (7) | 1.99 (1.23) | 1.92 | −0.13 (−0.64, 0.39) | 0.63 | −0.10 (−0.63, 0.42) | 0.70 |
| < 100% coverage | 399 (93) | 2.12 (1.38) | 2.08 | – | – | – | – |
| Other HH variables‡ | |||||||
| 2. Improved sanitation access of target HH | |||||||
| Improved | 103 (24) | 1.85 (1.25) | 1.60 | −0.37 (−0.67, −0.07) | 0.02 | −0.35 (−0.68, −0.03) | 0.03 |
| Unimproved | 325 (76) | 2.20 (1.40) | 2.20 | – | – | – | – |
| 3. Number of goat feces pile in compound | |||||||
| No feces | 295 (69) | 2.04 (1.36) | 1.90 | – | – | – | – |
| 1–10 piles | 90 (21) | 2.19 (1.26) | 2.20 | 0.18 (−0.14, 0.51) | 0.30 | 0.2 (−0.13, 0.52) | 0.24 |
| > 10 piles | 43 (10) | 2.50 (1.62) | 2.20 | 0.50 (0.06, 0.94) | 0.03 | 0.31 (−0.13, 0.76) | 0.17 |
| 4. Presence of any goat feces in HH¶ | 96 (22) | 2.37 (1.44) | 2.38 | 0.35 (0.04, 0.67) | 0.03 | – | – |
| 5. Number of cow dung pile in compound | |||||||
| No cow dung | 187 (44) | 2.06 (1.37) | 1.90 | 0 | – | ||
| 1–10 cow dung | 153 (36) | 2.15 (1.33) | 2.20 | 0.10 (−0.20, 0.39) | 0.52 | – | – |
| 10 > cow dung | 87 (20) | 2.17 (1.45) | 2.33 | 0.14 (−0.21, 0.49) | 0.43 | – | – |
| 6. Number of cow dung pile in HH | |||||||
| No cow dung | 248 (58) | 2.06 (1.37) | 1.90 | 0 | – | – | – |
| 1–10 cow dung | 129 (30) | 2.09(1.38) | 2.20 | 0.04 (−0.25, 0.32) | 0.79 | 0.07 (−0.23, 0.36) | 0.66 |
| 10 > cow dung | 51 (12) | 2.42 (1.34) | 2.45 | 0.36 (−0.05, 0.77) | 0.08 | 0.37 (−0.06, 0.79) | 0.09 |
| 7. Number of poultry feces piles in the compound | |||||||
| ≤ 10 piles | 219 (51) | 2.11 (1.35) | 2.08 | – | – | – | – |
| 10 > piles | 209 (49) | 2.12 (1.39) | 2.08 | 0.02 (−0.23, 0.28) | 0.85 | – | – |
| 8. Number of poultry feces piles in HH¶ | |||||||
| No feces | 91 (21) | 1.20 (1.24) | 1.90 | 0 | – | – | – |
| 1–10 piles | 208 (49) | 2.13 (1.46) | 2.08 | 0.14 (−0.21, 0.48) | 0.43 | – | – |
| More than 10 piles | 129 (30) | 2.17 (1.30) | 2.08 | 0.18 (−0.19, 0.56) | 0.33 | – | – |
| 9. Presence of appropriate water drainage | 247 (58) | 2.03 (1.35) | 1.90 | −0.21 (−0.48, 0.05) | 0.11 | −0.3 (−0.57, −0.03) | 0.03 |
| 10. Presence of an appropriate solid waste disposal system | 10 (2.3) | 1.78 (2.00) | 2.05 | −0.32 (−1.18, 0.53) | 0.46 | – | – |
| 11. Hands/nails looked visibly clean | 65 (15) | 1.88 (1.34) | 1.90 | −0.30 (−0.66, 0.06) | 0.10 | −0.23 (−0.59, 0.14) | 0.22 |
| 12. Mother with any formal education | 355 (83) | 2.07 (1.36) | 1.92 | −0.26 (−0.61, 0.08) | 0.13 | −0.23 (−0.59, 0.14) | 0.22 |
| 13. HH belongs to the upper (richest) wealth quintile | 78 (18) | 1.88 (1.19) | 1.70 | −0.34 (−0.68, −0.01) | 0.05 | −0.12 (−0.49, 0.24) | 0.52 |
| 14. Change in time (hour) of sample collection as the day progresses | – | – | – | −0.17 (−0.28, −0.07) | 0.002 | −0.17 (−0.28, −0.06) | 0.002 |
| 15. Study site | |||||||
| Narshingdi district | 226 (53) | 2.28 (1.38) | 2.20 | 0.34 (0.05, 0.63) | 0.02 | 0.49 (0.21, 0.76) | 0.001 |
| Mymensingh district | 216 (48) | 1.90 (1.34) | – | – | – | – | – |
CI = confidence intervals; HH = household; NHs = neighboring households.
* Number with presented category.
† Adjusting for clustering at village.
‡ The estimates and associated 95% CIs for the other HH variables presented here are from the multivariable model with variable 1a (proportion of improved sanitation coverage in the NH, as the primary outcome).
‖ The variables included in the multivariable model includes improved sanitation access in the target HH, number of goat feces pile in compound, number of cow dung pile in HH, presence of appropriate water drainage, hands/nails looked visibly clean, mother with any formal education, HH belongs to upper (richest) wealth quintile, change in time (hour) of sample collection as the day progress, and study site.
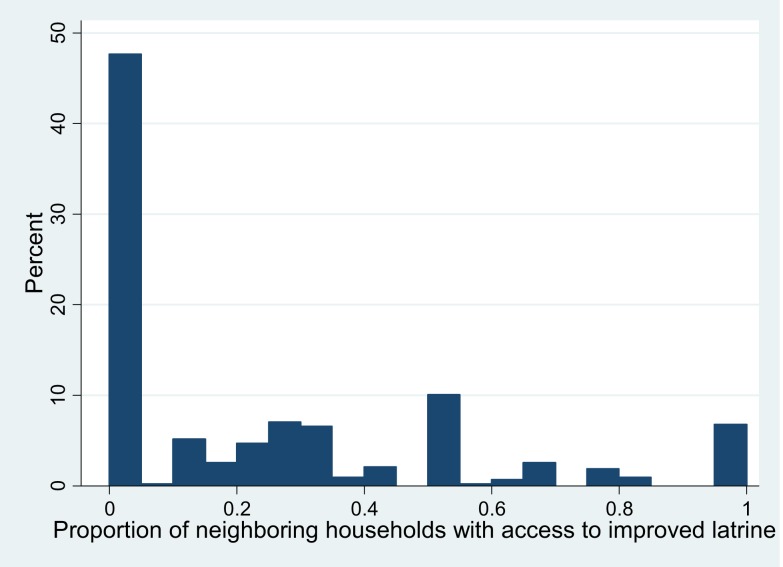
Almost half of the target households (n = 204, 48%) were from neighborhoods with no improved sanitation access. Seven percent of the target households (n = 29) were from neighborhoods with 100% improved sanitation coverage (Figure 3).
Figure 3.
Distribution of improved sanitation coverage in the neighborhood around 428 target households. This figure appears in color at www.ajtmh.org.
Fecal contamination of sentinel toy ball.
Among the 428 households where a toy ball was provided for the child to play with, mothers from only two households reported that the child did not play with the toy ball. Ninety-three percent (n = 399) of the mothers reported that the child played with the toy ball at least two times. Among the 428 sentinel toys, 44 (10%) of the rinse samples were below the detection limit for FCs. No samples had FC levels that were above the detection limit. On average, there were 2.11 (standard deviation [SD] = 1.37) log10 CFU/toy ball with a median of 2.08 log10 CFU/toy ball.
Toy ball samples collected from target households in neighborhoods with no private, improved sanitation access had 2.07 (SD = 1.45) log10 CFU/toy ball on average. There was minimal change in the level of toy ball contamination associated with higher improved sanitation coverage in the neighborhood. Toy ball samples collected from households in neighborhoods with less than 100% improved sanitation coverage had somewhat lower levels of contamination than households in neighborhoods with 100% improved sanitation coverage (difference in means = −0.13 log10 CFU/toy ball; 95% confidence intervals [CI]: −0.64, 0.39), but differences of this magnitude are consistent with random variation (P = 0.63). After adjusting for potential confounding household and neighborhood characteristics, the findings were similar (Table 2).
In restricted analysis among 103 target households with access to improved sanitation, a higher proportion of access to improved sanitation in the neighborhood was not associated with reduction in contamination of the toy ball with FCs. Even at 100% improved sanitation coverage in the neighborhood, there was minimal reduction in contamination of the toy ball with FCs (difference in mean = −0.03: 95% CI: −0.67, 0.60) compared with household with less than 100% improved sanitation access.
In the multivariate analysis, contamination of toy ball was lower in target households with access to private, improved sanitation compared with households with unimproved sanitation (adjusted difference in means = −0.35; 95% CI: −0.68, −0.03). Households with appropriate water drainage system had lower levels of FCs on toy ball than households without appropriate water drainage system (adjusted difference in means = −0.3, 95% CI: −0.57, −0.03). Toy rinse samples collected from households with more than 10 piles of cow dung had higher levels of FC contamination than children from households with no cow dung (adjusted difference in means = −0.37 log10 CFU/toy ball, 95% CI: −0.06, 0.79). However, the association was only weakly statistically significant (P = 0.09). Households from Narshingdi district had higher levels of FC contamination than households from Mymensingh district (adjusted difference in means = 0.49, 95% CI: 0.21, 0.76). Household wealth and mother’s education were not associated with FC level on hands (Table 2).
Fecal contamination of hands.
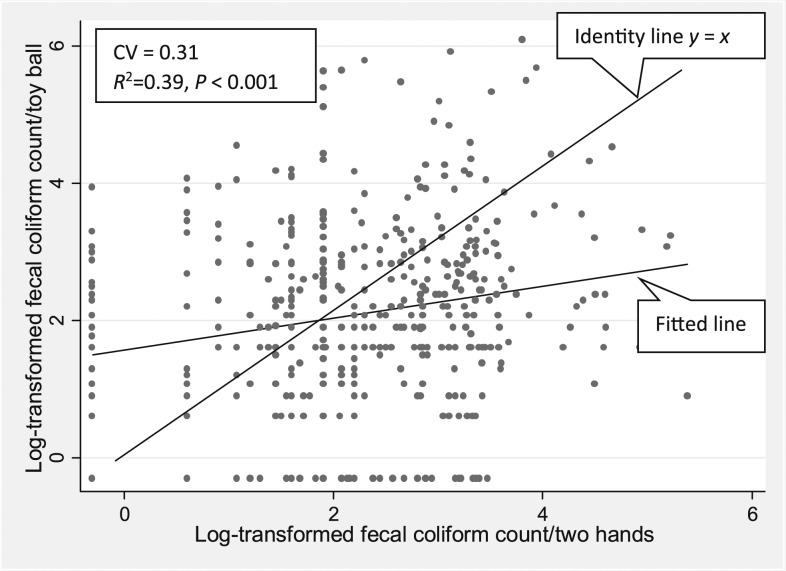
Among the hand rinse samples taken from 428 children younger than 2 years , 6% (n = 27) of the samples were below the detection limit for FCs. On average, children’s hands had 2.23 (SD 1.15) log10 CFU/two hands with a median of 2.20 log10 CFU/two hands. Hand contamination was only weakly correlated with toy ball contamination (r = 0.19, P = 0.44), and this low correlation could be due to chance alone (Figure 4). A one log10 increase in the level of FCs per two hands was associated with a 0.23 log10 increase in the level of FCs per sentinel toy ball (95% CI: 0.12, 0.34).
Figure 4.
Scatter plot showing log10-transformed fecal coliform contamination of children’s hands and toys in rural Bangladesh. CV = coefficient of variation.
In households from neighborhoods with no improved sanitation access, there were on average 2.26 (SD = 1.13) log10 CFU/two hands. There was no statistically significant change in the level of FCs on hands as coverage of improved sanitation in the neighborhood increased. Households in neighborhoods with 100% improved sanitation coverage had similar levels of hand contamination as those in neighborhoods with < 100% coverage (difference in means = −0.11; 95% CI: −0.53, 0.32) (Table 3).
Table 3.
Relationship between community-level water sanitation and hygiene-related variables and log10-transformed fecal coliform colony-forming units/two hands of children younger than 2 years in rural Bangladesh (N = 428 HHs)
| Exposures | n (%)* | Mean (standard deviation) | Median | Univariable | Multivariable | ||
|---|---|---|---|---|---|---|---|
| Difference in mean† (95% CI) | P-value† | Difference in mean† (95% CI) | P-value† | ||||
| NH improved sanitation coverage | |||||||
| 1a. Proportion of improved sanitation coverage in the NH¶ | |||||||
| No improved sanitation | 204 (48) | 2.26 (1.13) | 2.25 | – | – | – | – |
| Up to 25% coverage | 80 (19) | 2.28 (1.15) | 2.20 | −0.02 (−0.32, 0.28) | 0.89 | −0.04 (−0.33, 0.26) | 0.80 |
| 26 to 50% coverage | 88 (21) | 2.12 (1.28) | 2.30 | −0.16 (−0.44, 0.13) | 0.28 | −0.22 (−0.5, 0.06) | 0.13 |
| 51 to 100% coverage | 56 (13) | 2.25 (0.99) | 1.90 | −0.03 (−0.36, 0.31) | 0.87 | −0.06 (−0.41, 0.28) | 0.71 |
| 1b. Improved sanitation coverage in the NH¶ | |||||||
| 100% coverage | 29 (7) | 2.14 (1.04) | 1.90 | −0.11 (−0.53, 0.32) | 0.62 | −0.18 (−0.62, 0.25) | 0.41 |
| < 100% coverage | 399 (93) | 2.24 (1.16) | 2.20 | ||||
| Other confounding variables§ | |||||||
| 2. Improved sanitation access in the target HH | |||||||
| Improved | 103 (24) | 2.23 (1.13) | 2.20 | 0.02 (−0.23, 0.27) | 0.89 | 0.07 (−0.2, 0.34) | 0.61 |
| Unimproved | 325 (76) | 2.23 (1.16) | 2.20 | – | |||
| 3. Number of goat feces piles in compound | |||||||
| No feces | 295 (69%) | 2.18 (1.16) | 2.20 | – | |||
| 1 to 10 piles | 90 (21%) | 2.28 (1.12) | 2.20) | 0.09 (−0.18, 0.36) | 0.50 | 0.13 (−0.15, 0.4) | 0.37 |
| > 10 piles | 43 (10%) | 2.50 (1.11) | 2.86 | 0.31 (−0.06, 0.68) | 0.10 | 0.21 (−0.17, 0.58) | 0.29 |
| 4. Presence of any goat feces in HH | 96 (22) | 2.36 (1.13) | 2.30 | 0.14 (−0.12, 0.4) | 0.29 | ||
| 5. Number of cow dung piles in the compound | |||||||
| No cow dung | 187 (44%) | 2.21 (1.15) | 2.20 | – | |||
| 1 to 10 cow dung | 153 (36%) | 2.21 (1.13) | 2.20 | −0.01 (−0.26, 0.23) | 0.92 | ||
| 10 > cow dung | 87 (20%) | 2.31 (1.19) | 2.30 | 0.15 (−0.14, 0.44) | 0.31 | ||
| 6. Number of cow dung piles in the HH | |||||||
| No cow dung | 248 (58%) | 2.14 (1.13) | 2.20 | ||||
| 1 to 10 cow dung | 129 (30%) | 2.30 (1.19) | 2.30 | 0.13 (−0.11, 0.37) | 0.27 | 0.1 (−0.14, 0.35) | 0.42 |
| 10 > cow dung | 51 (12%) | 2.52 (1.08) | 2.78 | 0.46 (0.12, 0.8) | 0.01 | 0.42 (0.07, 0.78) | 0.02 |
| 7. Number of poultry feces piles in the compound | |||||||
| ≤ 10 piles | 219 (51%) | 2.23 (1.17) | 2.20 | – | |||
| 10 > piles | 209 (49%) | 2.23 (1.12) | 2.20 | −0.02 (−0.23, 0.2) | 0.89 | ||
| 8. Number of poultry feces piles in the HH | |||||||
| No feces | 91 (21%) | 2.10 (1.24) | 2.05 | ||||
| 1 to 10 piles | 208 (49%) | 2.29 (1.14) | 2.20 | 0.08 (−0.2, 0.37) | 0.57 | ||
| More than 10 piles | 129 (30%) | 2.23 (1.10) | 2.30 | 0.08 (−0.23, 0.39) | 0.62 | ||
| 9. Presence of appropriate water drainage | 247 (58%) | 2.23 (1.14) | 2.20 | 0.07 (−0.15, 0.29) | 0.52 | ||
| 10. Presence of an appropriate solid waste disposal system | 10 (2.34%) | 2.33 (1.36) | 2.25 | 0.16 (−0.55, 0.86) | 0.66 | ||
| 11. Target child washed hands within half an hour preceding hand rinse sample collection | 10 (2.34%) | 2.33 (1.36) | 2.25 | 0.16 (−0.55, 0.86) | 0.66 | ||
| 12. Child was active in the preceding half an hour (playing) | 339 (79%) | 2.30 (1.14) | 2.20 | 0.32 (0.06, 0.58) | 0.02 | 0.30 (0.04, 0.56) | 0.02 |
| 13. Mother with any formal education | 355 (83%) | 2.24 (1.15) | 2.20 | 0.07 (−0.21, 0.36) | 0.62 | 0.11 (−0.17, 0.4) | 0.44 |
| 14. HH belongs to upper (richest) wealth quintile | 78 (18%) | 2.07 (1.18) | 2.20 | −0.14 (−0.42, 0.14) | 0.33 | −0.18 (−0.47, 0.12) | 0.24 |
| 15. Change in time (hour) of sample collection as the day progress | −0.14 (−0.23, −0.05) | 0.004 | −0.14 (−0.23, −0.05) | 0.004 | |||
| 16. Study site—Narshingdi district | 226 (53%) | 2.19 (1.15) | 2.20 | −0.09 (−0.39, 0.21) | 0.56§ | ||
| Mymensingh district | 202 (47%) | 2.28 (1.14) | 2.30 | ||||
CI = confidence intervals; HH = household; NH = neighboring households.
* Number with presented category.
† Adjusting for clustering at village.
§ The estimates and associated 95% CIs for the other HH variables presented here are from the multivariable model with variable 1a (increase in improved sanitation coverage in the NH [as the primary outcome]).
¶ The variables included in the multivariable model includes improved sanitation access in the target HH, number of goat feces pile in compound, number of cow dung pile in HH, hands/nails looked visibly clean, child was active in the preceding half an hour (playing), mother with any formal education, HH belongs to upper (richest) wealth quintile, and change in time (hour) of sample collection as the day progress.
In the restricted analysis among target households with access to unimproved sanitation, a higher proportion of access to improved sanitation in the neighborhood was not associated with any reduction in FC level on children’s hands in the target household (data not shown).
In the multivariate analysis, hand contamination was similar in target households with access to private, improved sanitation and unimproved sanitation (adjusted difference in means = 0.07; 95% CI: −0.2, 0.34). Children, who were playing in the half hour preceding hand rinse sample collection, had higher levels of FCs than children who were inactive (e.g., sleeping) (adjusted difference in means = 0.30 log10 CFU/two hands, 95% CI: 0.04, 0.56). Children’s hands from households with more than 10 piles of cow dung had higher levels of FC contamination than children from households with no cow dung (adjusted difference in means = −0.42 log10 CFU/two hands, 95% CI: 0.07, 0.78). Household wealth and mother’s education were not associated with FC level on hands (Table 3).
DISCUSSION
In rural areas of Bangladesh with predominantly on-site sanitation, access to improved sanitation in NHs was associated with small and statistically insignificant reductions in fecal indicator bacteria on sentinel toy ball and children’s hands. Even 100% neighborhood coverage of improved sanitation was not associated with significant reduction in contamination. Access to private improved sanitation in the target household was associated with lower level of FCs on sentinel toy balls but not on children’s hands.
These findings suggest that in similar settings, high neighborhood coverage of improved sanitation may have little effect on target household’s level of fecal contamination.
There are several possible explanations as to why neighborhood sanitation coverage was not associated with levels of fecal indicator bacteria on children’s hands and toys. First, it is possible that household sanitation access influences household contamination levels more than neighborhood sanitation. Because children < 2 years of age are likely to spend most of their time within their own home, contamination of their hands and toys may closely reflect contamination of the their immediate domestic environment. In our study and in previous small-scale studies conducted in Bangladesh and Tanzania, household-level access to improved sanitation was found to be associated with lower contamination of toy balls and hands.26,41,42
Second, there are other routes of contamination, such as poor latrine cleanliness, presence of animal feces, or unsafe disposal of children’s feces, that neither target household nor neighborhood sanitation access can prevent. There may also be important community-level social, geographical, cultural, and/or environmental factors that we did not capture in our study because we found that sentinel toys in Narshingdi district had a higher level of contamination compared with children’s toys in Mymensingh district even after adjusting for potential confounding factors.
Third, it is possible that we were unable to detect a difference in the level of FCs associated with neighborhood sanitation because of low statistical power. Previous studies have found contamination on toys and hands to be highly variable requiring a large sample size to evaluate group differences.26 The sample size calculation for this study was not determined considering neighborhood sanitation coverage as the primary exposure. However, the finding that children who were sleeping had significantly lower levels of hand contamination than children who were playing and that children with visibly dirty hands had higher levels of hand contamination than children with visibly clean hands suggests that hands could be an important source of exposure to fecal contamination. The correlation between fecal contamination of sentinel toy balls and hands with fecal indicator bacteria was weak, and hand cleanliness was not associated with contamination of the toy ball. These findings suggest that contamination on toy balls is not a good indicator of hand contamination but contamination on toy ball could still be a good indicator of household environmental contamination that a child may come across. But from this study, we do not know if contamination on toy balls has biological relevance to child fecal exposure and human health. Future studies linking contamination on sentinel toys with human health could help determine their usefulness as an indicator of household fecal contamination.
Previous studies have identified neighborhood sanitation coverage as important in reducing diarrheal disease.10,14,43 Although we found only small and statistically insignificant effects of neighborhood sanitation coverage on sentinel balls and children’s hands, our results are not necessarily contradictory. Children in neighborhoods with higher coverage of improved sanitation may have experienced lower rates of diarrhea, despite similar measures of ball and hand contamination because other pathways of transmission (ingestion of contaminated food, water, and soil) may more strongly contribute to children’s exposure to fecal contamination. In our study, the presence of cow dung was associated with significantly higher contamination of children’s hands with fecal indicator bacteria, although the presence of goat feces and chicken feces was not associated with FC levels on hands or toy ball. In a previous study conducted in Bangladesh, mean FC contamination of toys increased as the number of animal feces piles observed in the household increased.26 Another study conducted in rural Bangladesh found that the presence of cow feces was associated with contamination of soil with fecal indicator bacteria but not with contamination of hands.44 A study conducted in India found that fewer toy balls were contaminated in households that had no animals.45 In a previous systematic review, heterogeneous effects of exposure to animals and animal feces on human health were observed.46 This suggests that separation of human feces from human contact may not be sufficient to reduce exposure to diarrhea causing enteric pathogens. More research needs to be carried out to understand how separation of children and animal feces can be achieved in settings where ownership of domestic animal is common.
An important limitation of this study is the use of fecal indicator bacteria to assess fecal contamination, which may not be correlated with the presence of a wide range of pathogens, including viruses.47 Moreover, fecal indicator bacteria may have nonhuman origin and does not necessarily signify risks to human health.42,48–54 In the study households, several types of animal feces were observed. This makes presence of fecal indicator bacteria an imprecise outcome indicator for sanitation because latrines are used for confining human feces and not for animal. This measurement error can introduce bias because of misclassification of outcome. As a consequence, the confidence intervals of the estimates presented are likely to be wider, making the results less likely to be statistically significant even if in reality, they are statistically significant.55 For example, having 100% improved sanitation access in the neighborhood was associated with lower but statistically insignificant reduction in the level of fecal contamination. So, further study with a larger sample size could clarify the role of neighborhood sanitation.55 Using molecular markers of human specific pathogens as indicator of FCs could help reduce this bias in future studies. However, the use of fecal indicator bacteria is common practice and still provides useful comparative information about overall differences in bacterial loading on hands and surfaces in study households.
Finally, our definition of neighborhood may be problematic. The cutoff point of a 20-m radius was arbitrary, based on logistical convenience and high population density in this context rather than scientific evidence. So, our conclusion may be conservative given small radius. Selecting a larger radius might have resulted in a different conclusion. Moreover, there may be issues with generalizability of these findings. Bangladesh has high water tables and high number of domestic animals; as a result, Bangladesh may have many determinants of household fecal contamination that are not impacted on by neighborhood sanitation practices.
Neighborhood coverage with improved sanitation within 20 m of households in rural Bangladesh had no association with fecal contamination of the household environment, measured as indicator bacteria on children’s hands and toys. In this context, household sanitation access is probably more important than neighborhood sanitation coverage in preventing fecal contamination of domestic environment. Household factors such as absence of cow dung, presence of appropriate water drainage, and optimal handwashing practices of children may be more important in reducing contamination of the household environment that the child may be exposed to. Intervention studies with larger sample sizes might help us better understand the impact of neighborhood sanitation coverage on fecal contamination of household environments.
Acknowledgments:
This research was made possible with UK Aid from the Department of International Development (DFID) as part of the SHARE research program (www.SHAREresearch.org) and through UNICEF Bangladesh, grant number GR00828. However, the views expressed do not necessarily reflect DFID’s official policies. This research was financially supported [in part] by Grant OPPGD759 from the Bill & Melinda Gates Foundation to the University of California, Berkeley. No funding bodies had any role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. icddr,b acknowledges with gratitude the commitment of DFID and Bill & Melinda Gates Foundation to its research efforts. icddr,b is also grateful to the governments of Bangladesh, Canada, Sweden, and the UK for providing core/unrestricted support. The authors acknowledge the contributions of the study participants, and icddr,b admin, field, and laboratory research staff. The authors are thankful to Amal Krishna Halder, Md. Mahbubur Rahman, and Sharker Masud Parvez from icddr,b for their logistic support during the field activities; the research advisory committee and the examiners, the EHG group at LSHTM; Ben Arnold and Jade Benjamin-Chung from UC Berkeley, CA; and Jane Bruce from LSHTM for statistical advice.
REFERENCES
- 1.Kawata K, 1978. Water and other environmental interventions—the minimum investment concept. Am J Clin Nutr 31: 2114–2123. [DOI] [PubMed] [Google Scholar]
- 2.Wagner EG, Lanoix JN, 1958. Excreta disposal for rural areas and small communities. Monogr Ser World Health Organ 39: 1–182. [PubMed] [Google Scholar]
- 3.Curtis V, Cairncross S, Yonli R, 2000. Domestic hygiene and diarrhoea—pinpointing the problem. Trop Med Int Health 5: 22–32. [DOI] [PubMed] [Google Scholar]
- 4.Cutting WAM, 1991. Diarrhoeal diseases. Stanfield P, Brueton M, Chan M, Parkin M, Waterston T, eds. Diseases of Children in the Subtropics and Tropics. London, United Kingdom: Edward Arnold. [Google Scholar]
- 5.Foster AD, 1993. Household partition in rural Bangladesh. Popul Stud 47: 97–114. [Google Scholar]
- 6.Pinfold JV, 1990. Fecal contamination of water and fingertip-rinses as a method for evaluating the effect of low-cost water supply and sanitation activities on faeco-oral disease transmission. II. A hygiene intervention study in rural north-east Thailand. Epidemiol Infect 105: 377–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Curtis V, Biran A, Deverell K, Hughes C, Bellamy K, Drasar B, 2003. Hygiene in the home: relating bugs and behaviour. Soc Sci Med 57: 657–672. [DOI] [PubMed] [Google Scholar]
- 8.Cairncross S, Blumenthal U, Kolsky P, Moraes L, Tayeh A, 1996. The public and domestic domains in the transmission of disease. Trop Med Int Health 1: 27–34. [DOI] [PubMed] [Google Scholar]
- 9.Andres LA, Briceno B, Chase C, Echenique JA, 2014. Sanitation and Externalities: Evidence from Early Childhood Health in Rural India. Policy Research Working Paper 6737 Washington, DC: World Bank. [Google Scholar]
- 10.Root GP, 2001. Sanitation, community environments, and childhood diarrhoea in rural Zimbabwe. J Health Popul Nutr 19: 73–82. [PubMed] [Google Scholar]
- 11.Lopez AL, MacAsaet LY, Ylade M, Tayag EA, Ali M, 2015. Epidemiology of cholera in the Philippines. PLoS Negl Trop Dis 9: e3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Penrose K, De Castro MC, Werema J, Ryan ET, 2010. Informal urban settlements and cholera risk in Dar es Salaam, Tanzania. PLoS Negl Trop Dis 4: e631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barreto ML, et al. 2010. Impact of a citywide sanitation program in northeast Brazil on intestinal parasites infection in young children. Environ Health Perspect 118: 1637–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barreto ML, et al. 2007. Effect of city-wide sanitation programme on reduction in rate of childhood diarrhoea in northeast Brazil: assessment by two cohort studies. Lancet 370: 1622–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.VanDerslice J, Briscoe J, 1995. Environmental interventions in developing countries: interactions and their implications. Am J Epidemiol 141: 135–144. [DOI] [PubMed] [Google Scholar]
- 16.Mollah KA, Aramaki T, 2010. Social-epidemiological study for evaluation of water supply and sanitation systems of low-income urban community in Dhaka, Bangladesh. J Water Health 8: 184–191. [DOI] [PubMed] [Google Scholar]
- 17.WHO/UNICEF JMP , 2015. 25 Years Progress on Sanitation and Drinking-Water—2015 Update and MDG Assessment. Geneva, Switzerland: WHO/UNICEF Joint Monitoring Programme for Water Supply and Sanitation. [Google Scholar]
- 18.Goldstick JE, Trostle J, Eisenberg JNS, 2014. Ask when—not just whether—it’s a risk: how regional context influences local causes of diarrheal disease. Am J Epidemiol 179: 1247–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spears D, 2013. How Much International Variation in Child Height Can Sanitation Explain? Policy Research Working Paper 6351 Washington, DC: The World Bank. [Google Scholar]
- 20.Fuller JA, Clasen T, Heijnen M, Eisenberg JN, 2014. Shared sanitation and the prevalence of diarrhea in young children: evidence from 51 countries, 2001–2011. Am J Trop Med Hyg 91: 173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fuller JA, Westphal JA, Kenney B, Eisenberg JNS, 2015. The joint effects of water and sanitation on diarrhoeal disease: a multicountry analysis of the demographic and health surveys. Trop Med Int Health 20: 284–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huda TM, Unicomb L, Johnston RB, Halder AK, Yushuf Sharker MA, Luby SP, 2012. Interim evaluation of a large scale sanitation, hygiene and water improvement programme on childhood diarrhea and respiratory disease in rural Bangladesh. Soc Sci Med 75: 604–611. [DOI] [PubMed] [Google Scholar]
- 23.Huda TMN, Schmidt WP, Pickering AJ, Mahmud ZH, Islam MS, Rahman MS, Luby SP, Biran A, 2018. A cross sectional study of the association between sanitation type and fecal contamination of the household environment in rural Bangladesh. Am J Trop Med Hyg 98: 967–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.GARMIN, eTrex Legend® H, 2017. Available at: https://buy.garmin.com/en-GB/GB/outdoor/discontinued/etrex-legend-h/prod30120.html. Accessed June 29, 2017.
- 25.Larson EL, Strom MS, Evans CA, 1980. Analysis of three variables in sampling solutions used to assay bacteria of hands: type of solution, use of antiseptic neutralizers, and solution temperature. J Clin Microbiol 12: 355–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vujcic J, Ram PK, Hussain F, Unicomb L, Gope PS, Abedin J, Mahmud ZH, Islam MS, Luby SP, 2014. Toys and toilets: cross-sectional study using children’s toys to evaluate environmental fecal contamination in rural Bangladeshi households with different sanitation facilities and practices. Trop Med Int Health 19: 528–536. [DOI] [PubMed] [Google Scholar]
- 27.CDC , 2010. Microbiological Indicator Testing in Developing Countries: A Fact Sheet for the Field Practitioner. Available at: http://sanitationupdates.files.wordpress.com/2010/11/microbiology2020.pdf. Accessed September 12, 2013. [Google Scholar]
- 28.USEPA , 2002. Method 1604: Total Coliforms and Escherichia coli in Water by Membrane Filtration Using a Simultaneous Detection Technique (MI Medium). Washington, DC: US Environmental Protection Agency. [Google Scholar]
- 29.WHO/UNICEF JMP , 2014. Progress on Sanitation and Drinking-Water—2014 Update. Geneva, Switzerland: WHO/UNICEF Joint Monitoring Programme for Water Supply and Sanitation. [Google Scholar]
- 30.Luby SP, Halder AK, Huda T, Unicomb L, Johnston RB, 2011. The effect of handwashing at recommended times with water alone and with soap on child diarrhea in rural Bangladesh: an observational study. PLoS Med 8: e1001052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vyas S, Kumaranayake L, 2006. Constructing socio-economic status indices: how to use principal components analysis. Health Policy Plan 21: 459–468. [DOI] [PubMed] [Google Scholar]
- 32.Barros AJ, Victora CG, 2005. A nationwide wealth score based on the 2000 Brazilian demographic census. Rev Saude Publica 39: 523–529. [DOI] [PubMed] [Google Scholar]
- 33.Houweling TA, Kunst AE, Mackenbach JP, 2003. Measuring health inequality among children in developing countries: does the choice of the indicator of economic status matter? Int J Equity Health 2: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.WHO/UNICEF , 2006. Core Questions on Drinking-Water and Sanitation for Household Surveys. Available at: http://www.who.int/water_sanitation_health/monitoring/oms_brochure_core_questionsfinal24608.pdf. Accessed September 12, 2013. [Google Scholar]
- 35.UCLA: Statistical Consultating Group , 2014. Analyzing Correlated (Clustered) Data. Available at: http://www.ats.ucla.edu/stat/stata/library/cpsu.htm. Accessed August 25, 2014. [Google Scholar]
- 36.UCLA: Statistical Consultating Group , 2014. What is the Relationship between xtreg-re, xtreg-fe and xtreg-be? Available at: http://www.ats.ucla.edu/stat/stata/faq/revsfe.htm. Accessed August 25, 2014. [Google Scholar]
- 37.UCLA: Statistical Consulting Group , 2010. What are the Some of the Methods for Analyzing Clustered Data in Stata? Available at: http://www.ats.ucla.edu/stat/STATA/faq/clusterreg.htm. Accessed October 23, 2010. [Google Scholar]
- 38.Greene WH, 1997. Econometric Analysis. Upper Saddle River, NJ: Prentice Hall. [Google Scholar]
- 39.Kirkwood BR, Sterne JAC, 2003. Essential Medical Statistics. Oxford, United Kingdom: Blackwell Science, Ltd. [Google Scholar]
- 40.Greenland S, Pearl J, Robins JM, 1999. Causal diagrams for epidemiologic research. Epidemiology 10: 37–48. [PubMed] [Google Scholar]
- 41.DiVita MA, Halder AK, Jahid IK, Islam M, Sobsey MD, Luby SP, Ram PK, 2008. The Utility of Common Household Objects as Markers of Home Hygiene in the Context of Access to Improved Sanitation. ISEE 20th Annual Conference, Pasadena, CA, October 12–16, 2008. [Google Scholar]
- 42.Pickering AJ, et al. 2010. Hands, water, and health: fecal contamination in Tanzanian communities with improved, non-networked water supplies. Environ Sci Technol 44: 3267–3272. [DOI] [PubMed] [Google Scholar]
- 43.Aziz KM, Hoque BA, Hasan KZ, Patwary MY, Huttly SR, Rahaman MM, Feachem RG, 1990. Reduction in diarrhoeal diseases in children in rural Bangladesh by environmental and behavioural modifications. Trans R Soc Trop Med Hyg 84: 433–438. [DOI] [PubMed] [Google Scholar]
- 44.Ercumen A, et al. 2017. Animal feces contribute to domestic fecal contamination: evidence from E. coli measured in water, hands, food, flies, and soil in Bangladesh. Environ Sci Technol 51: 8725–8734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Torondel B, Gyekye-Aboagye Y, Routray P, Boisson S, Schimdt W, Clasen T, 2015. Laboratory development and field testing of sentinel toys to assess environmental fecal exposure of young children in rural India. Trans R Soc Trop Med Hyg 109: 386–392. [DOI] [PubMed] [Google Scholar]
- 46.Penakalapati G, Swarthout J, Delahoy MJ, McAliley L, Wodnik B, Levy K, Freeman MC, 2017. Exposure to animal feces and human health: a systematic review and proposed research priorities. Environ Sci Technol 51: 11537–11552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tyagi VK, Chopra AK, Kazmi AA, Kumar A, 2006. Alternative microbial indicatrosd or fecal pollution: current perspective. Iran J Environ Health Sci Eng 3: 205–216. [Google Scholar]
- 48.Rajal VB, Cruz C, Last JA, 2010. Water quality issues and infant diarrhoea in a South American province. Glob Public Health 5: 348–363. [DOI] [PubMed] [Google Scholar]
- 49.Scott TM, Rose JB, Jenkins TM, Farrah SR, Lukasik J, 2002. Microbial source tracking: current methodology and future directions. Appl Environ Microbiol 68: 5796–5803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.USEPA , 2006. Voluntary Estuary Monitoring Manual Chapter 17: Bacteria Indicators of Potential Pathogens. Available at: http://water.epa.gov/type/oceb/nep/upload/2009_03_13_estuaries_monitor_chap17.pdf. Accessed August 13, 2013. [Google Scholar]
- 51.Bermudez M, Hazen TC, 1988. Phenotypic and genotypic comparison of Escherichia coli from pristine tropical waters. Appl Environ Microbiol 54: 979–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hazen TC, 1988. Fecal coliforms as indicators in tropical waters: a review. Environ Toxicol 3: 461–477. [Google Scholar]
- 53.Desmarais TR, Solo-Gabriele HM, Palmer CJ, 2002. Influence of soil on fecal indicator organisms in a tidally influenced subtropical environment. Appl Environ Microbiol 68: 1165–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sobsey MD, Schwab KJ, Handzel TR, 1990. Simple membrane filter method to concentrate and enumerate male-specific RNA coliphages. J Am Water Works Assoc 82: 52–59. [Google Scholar]
- 55.Hutcheon JA, Chiolero A, Hanley JA, 2010. Random measurement error and regression dilution bias. BMJ 340: c2289. [DOI] [PubMed] [Google Scholar]