Figure 3. Requirements for DYRK1A and SMAD signaling.
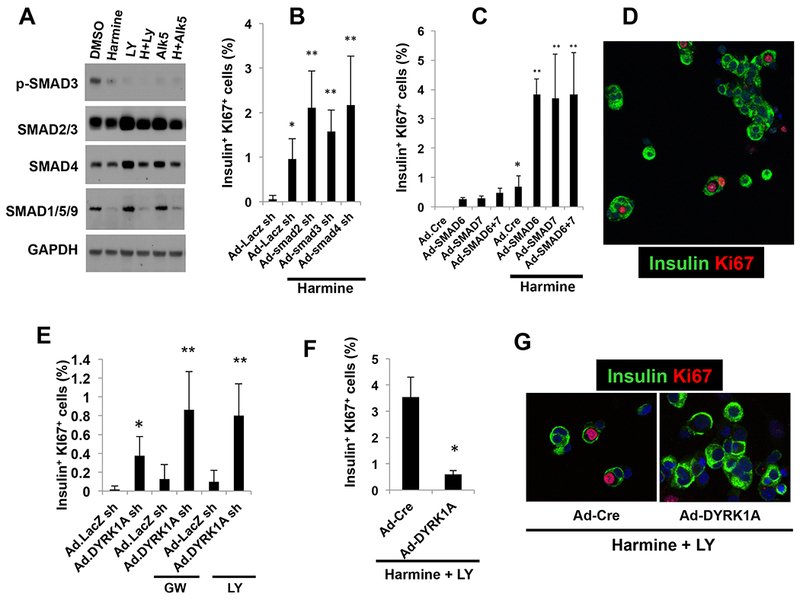
A. Immunoblots of control, harmine-, LY364947-, ALK5 inhibitor- or combination-treated whole human islets. While SMAD2 and SMAD3 (detected by the same antibody) did not change, p-SMAD3 was reduced by harmine, and further reduced by LY364947 or ALK5 inhibitor and the combinations. SMAD1, 5 and 9 are also detected by a common antibody, and are reduced by harmine and the drug combinations. The immunoblots are representative of separate experiments in human islets from three different donors. B. Effects of a control adenovirus expressing an shRNA directed against LacZ and adenoviruses silencing SMAD2,3 and 4 (150 moi each) on Ki67 immunolabeling in harmine-treated human islets. C. Effects of adenoviral SMAD6 and SMAD7 overexpression (100 moi each) on beta cell proliferation, alone and in combination with harmine. D. An example of the mitogenic effects of SMAD7 silencing in human beta cells (green) on Ki67 immunolabeling. E. The effects of adenoviral silencing of DYRK1A in combination with TGFβSF inhibitors GW788388 or LY364947. Ad.shLacZ indicates a control sh-adenovirus for the Ad.shDYRK1A. F. Effect of adenoviral DYRK1A overexpression or a control adenovirus expressing Cre (Ad.Cre) on proliferation in human islets treated with the harmine-LY364947 combination. G. Examples of Ad.Cre- and Ad.DYRK1A-overexpressing viruses on Ki67 immunolabeling in human islets treated with harmine and LY364947. All adenovirus experiments were for 96 hours, and all experiments were done on dispersed islets. In all panels, error bars represent mean ± SEM, *indicates p<0.05 vs. control, and **indicates p<0.05 vs. harmine. Numbers of donors and beta cells counted are provided in Supplemental Table 3.
