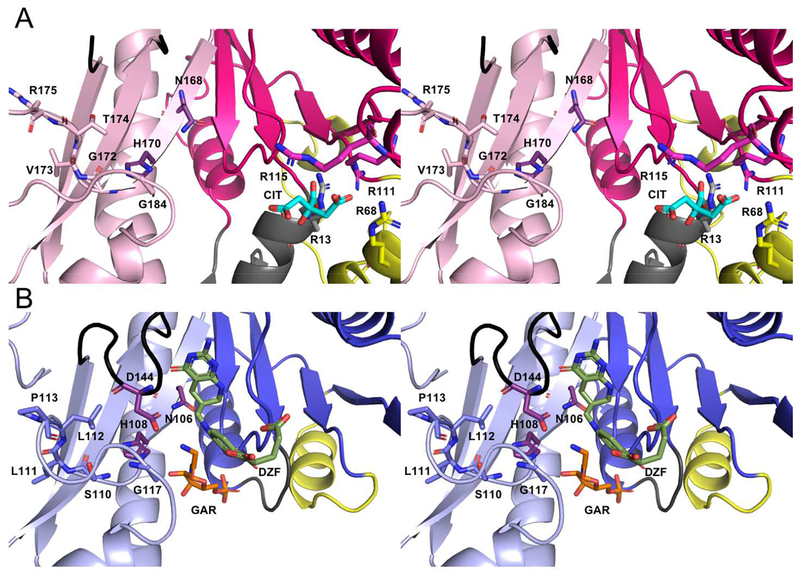
Figure 5.
Walleye stereo comparison of the active sites of PvdF (A) and EcGART (B). EcGART contains the folate binding motif (S110-P113, light blue), that is not conserved in PvdF (G172-R175, light pink). The catalytic triad of Asn, His and Asp (shown in purple) is conserved in both PvdF and EcGART with Asp being part of the folate binding loop (shown in black). This loop was mobile in PvdF and not resolved. Because of unique insertions (gray and yellow, Panel A), PvdF contains an arginine binding pocket that contains a citrate molecule (cyan) derived from the crystallization mother liquor. By comparison to the binding orientation of the THF analogue in GART (dark green, Panel B), we propose that this arginine pocket may stabilize the mobile glutamate tail of the folate analogue in PvdF during catalysis.