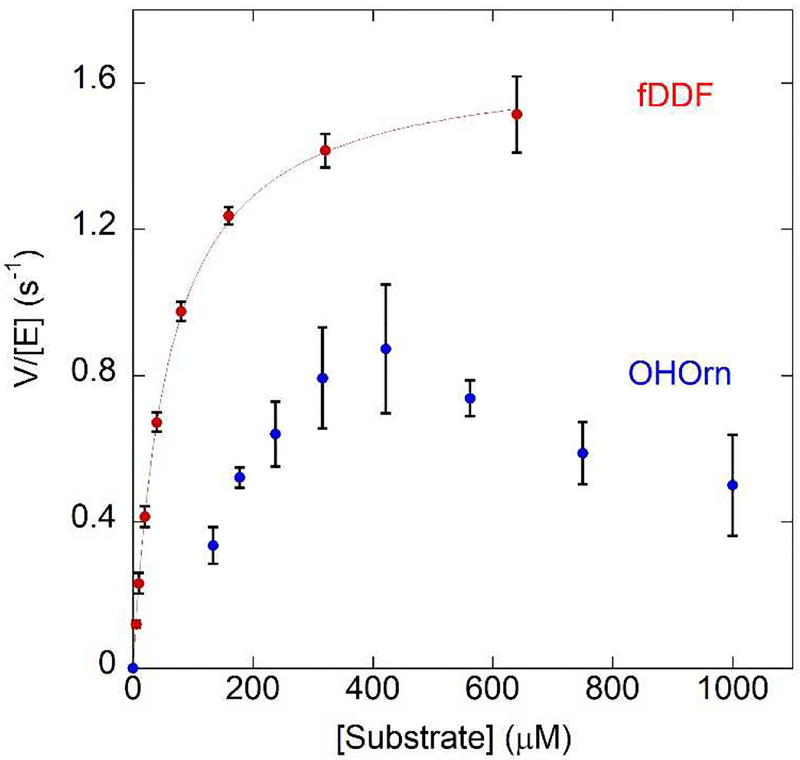
Figure 6.
In the presence of fDDF as the varied substrate PvdF shows typical Michaelis-Menten kinetics (red trace, Km = 60 ± 10 μM, kcat = 1.7 ± 0.1 sec−1). In this experiment, the synthesized OHOrn was used. Higher concentrations of fDDF were not possible due to the highly absorbant nature of the compound exceeding the linear range of the spectrometer. Using hydroxyornithine as the varied substrate generated by PvdA in a coupled assay, PvdF initially exhibits a sigmoidal curve (blue dotted trace) until 400 uM, after which the rate decreases. The model that best describes this behavior is a random bireactant mechanism in which the binding of fDDF is preferred first step