Table 1.
Optimization of CuH-catalyzed enantioselective N- and C3-alkylation of indole derivatives.a
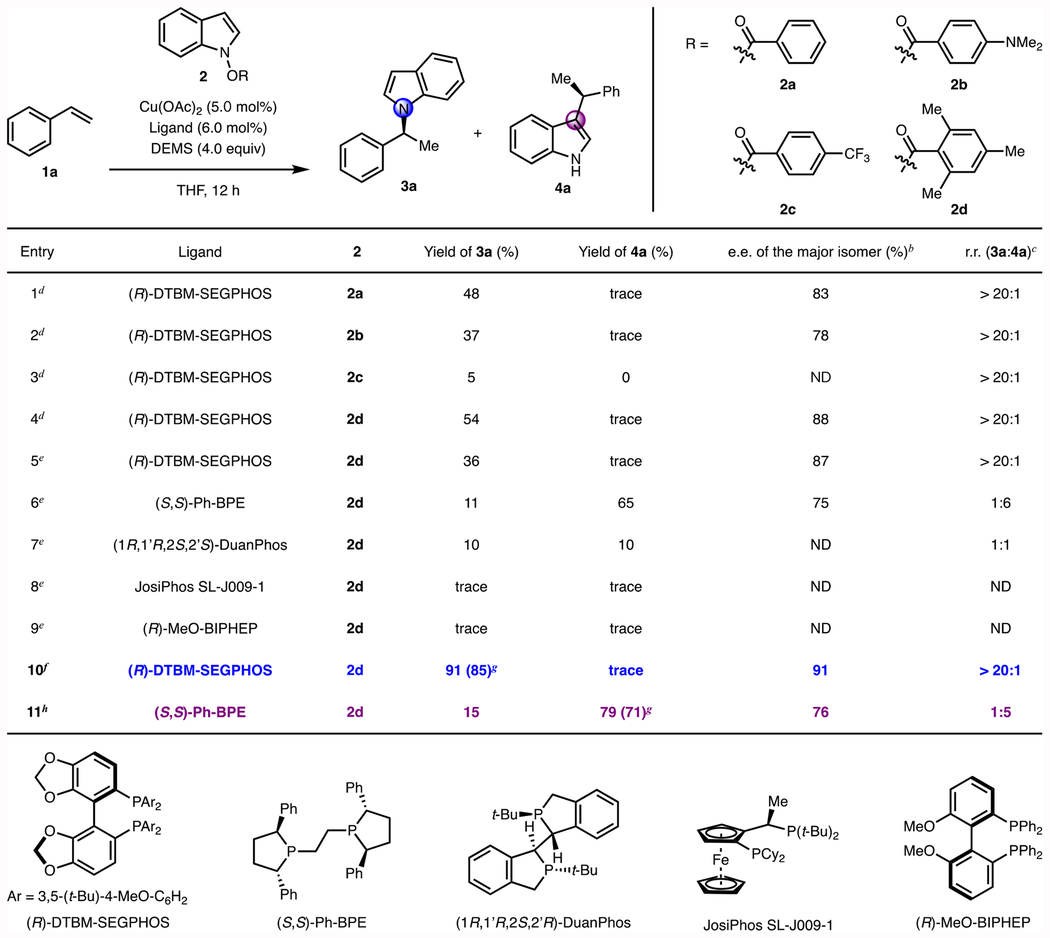
|
Reactions were conducted on 0.10 mmol scale. Yields were determined by gas chromatography using dodecane as internal standard.
The ee was determined by SFC analysis.
The regioisomeric ratio (rr) was determined by GC analysis of the crude reaction mixture.
Conditions: 1 (0.10 mmol), 2 (0.10 mmol), Cu(OAc)2 (5.0 mol%), (R)-DTBM-SEGPHOS (6.0 mol%), DEMS (4.0 equiv), THF (0.1 M), 90 °C, 12 h.
Conditions: 1 (0.15 mmol), 2 (0.10 mmol), Cu(OAc)2 (5.0 mol%), ligand (6.0 mol%), DEMS (4.0 equiv), THF (0.5 M), 50 °C, 12 h.
Conditions: 1 (0.10 mmol), 2 (0.15 mmol), Et3COH (0.02 mmol), Cu(OAc)2 (5.0 mol%), (R)-DTBM-SEGPHOS (6.0 mol%), DEMS (4.0 equiv), THF (0.1 M), 90 °C, 20 h.
Isolated yield on a 0.50 mmol scale.
Conditions: 1 (0.20 mmol), 2d (0.10 mmol), Et3COH (0.02 mmol), Cu(OAc)2 (1.0 mol%), (S,S)-Ph-BPE (1.2 mol%), DMMS (4.0 equiv), THF (0.5 M), 40 °C, 24 h. ND = Not determined. DEMS = (EtO)2MeSiH. DMMS = (MeO)2MeSiH.
