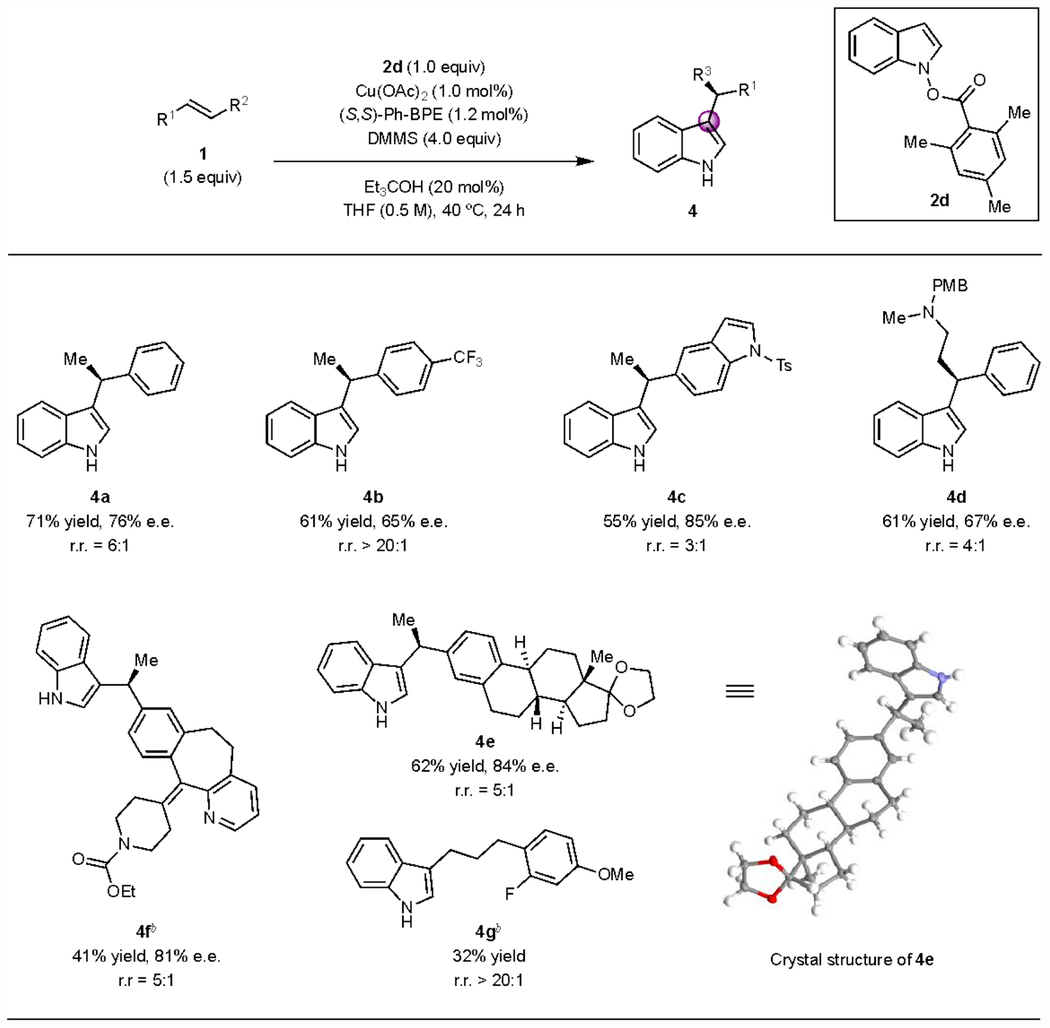
Table 3.
Substrate scope of CuH-catalyzed enantioselective C3-alkylation.a
|
Conditions: 1 (1.0 mmol), 2d (0.50 mmol), Et3COH (0.10 mmol), Cu(OAc)2 (1.0 mol%), (S,S)-Ph-BPE (1.2 mol%), DMMS (4.0 equiv), THF (0.5 M), 40 °C, 24 h. The ee was determined by SFC analysis. The regioisomeric ratio (rr) was determined by 1H NMR analysis of the crude reaction mixture. DMMS = (MeO)2MeSiH.
Using 5.0 mol% Cu(OAc)2 and 6.0 mol% (S,S)-Ph-BPE instead of 1.0 mol% Cu(OAc)2 and 1.2 mol% (S,S)-Ph-BPE.
