Abstract
Background
Psychosis risk is associated with striatal dysfunction, including a previous behavioral study that found that psychosis risk is associated with impaired performance on a probabilistic category learning task (PCLT; ie, the Weather Prediction Task), a task strongly associated with striatal activation. The current study examined whether psychosis risk based on symptom levels was associated with both poor behavioral performance and task-related physiological dysfunction in specific regions of the striatum while performing the PCLT.
Methods
There were 2 groups of participants: psychosis risk (n = 21) who had both (a) extreme levels of self-reported psychotic-like beliefs and experiences and (b) interview-rated current attenuated psychotic symptoms (APS); and a comparison group (n = 20) who had average levels of self-reported psychotic-like beliefs and experiences. Participants completed the PCLT during fMRI scanning.
Results
The current research replicated previous work finding behavioral PCLT deficits at the end of the task in psychosis risk. Furthermore, as expected, the psychosis risk group exhibited decreased striatal activation on the task, especially in the associative striatum. The psychosis risk group also displayed decreased activation in a range of cortical regions connected to the associative striatum. In contrast, the psychosis risk group exhibited greater activation predominantly in cortical regions not connected to the associative striatum.
Conclusions
Psychosis risk was associated with both behavioral and striatal dysfunction during performance on the PCLT, suggesting that behavioral and imaging measures using this task could be a marker for psychosis risk.
Keywords: psychosis risk, attenuated psychotic symptoms, probabilistic category learning, fMRI, striatum, associative striatum
Psychotic disorders, involving the symptoms of delusions and hallucinations, are commonly preceded by a prodromal period characterized as reflecting an at-risk mental state (ARMS). ARMS is often defined by the presence of basic symptoms (BS; ie, early disturbances in cognitive and perceptual processing), brief and self-limiting psychotic symptoms (BLIPS), a significant decrease in functioning in the context of genetic risk for schizophrenia (GRD), or attenuated psychotic symptoms (ie, subthreshold delusions and hallucinations). There is evidence that individuals with both elevated psychosis proneness questionnaire scores and APS are at increased risk of psychotic disorder, with 14% developed a psychotic disorder in 10 years1 and an estimated lifetime risk of psychotic disorder >20%,2 a lifetime rate that is at least comparable to the rate of psychotic disorder in first-degree relatives.3 A well-replicated neurobiological correlate of ARMS4 (eg, BS and APS5,6) and increased genetic risk7 is striatal dysfunction. Psychosis risk is associated with both striatum-related learning deficits8–12 and impaired striatal activation.5–7,13–16 The current study examined whether psychosis risk based on symptom levels was associated with both poor behavioral performance and task-related physiological dysfunction in the striatum.
The striatum is the input layer of the basal ganglia17 and is critically involved in corticostriatal circuits, or the functional loops composed of striatal regions projecting to the thalamus, which in turn projects to cortical regions, and then back to the striatum.18 Parcellation research indicates that different striatal subregions can be distinguished based on white matter and resting state functional connectivity.19 Furthermore, in most recent in vivo brain imaging studies examining dopamine in psychosis risk, researchers have examined striatal dysfunction in particular regions of the striatum. This research has most consistently found striatal dysfunction in the associative striatum in psychosis risk,4,20–22 including evidence of decreased functional connectivity between the associative striatum with cortical areas.23 Some research, including a recent meta-analysis,13 has localized this impairment in the ventral striatum (ie, nucleus accumbens),5,6 although several recent studies having failed to find ventral striatal dysfunction.23,24 The current research aimed to examine whether psychosis risk based on symptom levels was associated with striatal impairment in a particular region of the striatum.
A behavioral task that strongly activates the associative striatum is the Probabilistic Category Learning Task (PCLT).25–27 This suggests that psychosis risk might be associated with impaired performance on the PCLT. We recently found that psychosis risk (but not elevated negative symptoms) was strongly associated with poor performance on the PCLT,8 consistent with PCLT research in individuals with increased genetic risk7,10,12 and individuals with elevated schizotypal traits.9 Furthermore, research indicates that genetic risk for schizophrenia is associated with impaired striatal activation on the PCLT.7 However, the current study is the first to examine whether psychosis risk based on symptom levels (ie, not in those at increased genetic risk)7 in antipsychotic medication-naïve participants was associated with both poor performance and dysfunction in specific striatal regions on the PCLT.
Materials and Methods
Participants
Participants provided informed consent before completing the study. Ethical research procedures were approved by the University of Missouri’s Institutional Review Board. Participants were undergraduate students recruited as in previous research (see the supplementary methods for more information about the overall sample from which participants were recruited).8,28 Potential participants were excluded if they did not meet inclusion criteria (detailed below and in supplementary methods), or were under age 18, not fluent in English, or had an MRI contraindication (eg, irremovable ferromagnetic implants). People in the psychosis risk group (see table 1 for sample characteristics; n = 21) had both (a) extreme elevation on psychosis proneness questionnaires, ie, >1.96 SD above the same sex mean on the Perceptual Aberration (PerAb)29 or Magical Ideation (MagicId)30 scales, or a summed standardized score 3.0 SDs above the same sex mean on the PerAb and MagicId scales; and (b) interview-rated current attenuated psychotic symptoms (APS; ie, a rating of at least moderate on the Structured Interview for Prodromal Syndromes, (SIPS)],31 with all participants exhibiting APS in the past month (in fact, all within the past week). This combined questionnaire and interview approach was used because while self-reported psychosis proneness is associated with psychosis risk,32 there is evidence that actually only those individuals with both elevated psychosis proneness questionnaire scores and interview-rated APS are at increased risk of psychotic disorder.1 In the current study, all participants in the psychosis risk group also reported never having taken antipsychotic medication. Hence, the psychosis risk group in the current study had current APS, was antipsychotic medication-naïve, and had a level of psychotic disorder risk comparable to a first-degree relative sample. Note that one participant in the psychosis risk group was excluded from brain imaging analyses due to excessive artifacts, leaving 20 participants in the psychosis risk group for the brain imaging analyses (this subject is included in behavioral data analyses).
Table 1.
Sample Characteristics of Participantsa
| Psychosis Risk Group (n = 21) | Comparison Group (n = 20) | P-Value | |
|---|---|---|---|
| Race/ethnicityb | .058 | ||
| White (%) | 66.7% | 90.0% | |
| African American (%) | 28.6% | — | |
| Asian American (%) | 4.8% | 5.0% | |
| Biracial (%) | — | 5.0% | |
| Age | 18.29 (0.56) | 18.35 (0.59) | .72 |
| Year of education | 13.14 (0.36) | 13.10 (0.31) | .68 |
| Sex (% female) | 71.4% | 70.0% | .92 |
| Handedness (% left-handed)c | 9.5% | 5% | .58 |
| Psychotropic medication use (%)d | 23.8% | 15.0% | .48 |
| Stimulants | 9.5% | 5.0% | .58 |
| Antidepressants | 19.0% | 10.0% | .41 |
| Anxiolytics | 14.3% | 5.0% | .32 |
| Wisconsin schizotypy scales | |||
| Magical ideation | 20.05 (3.41) | 7.80 (1.58) | <.001 |
| Perceptual aberration | 16.90 (6.53) | 4.15 (1.35) | <.001 |
| SIPS scalese (% APS) | |||
| Unusual thought content | 71.43% | 0.00% | <.001 |
| Suspiciousness | 38.10% | 0.00% | <.001 |
| Grandiosity | 9.52% | 0.00% | <.001 |
| Perceptual aberrations | 66.67% | 0.00% | <.001 |
| Disorganized communication | 33.33% | 0.00% | <.001 |
Note. SIPS, Structured Interview for Prodromal Syndromes.
aMeans (SD). Independent samples t-tests were used to compare means for the psychosis risk and comparison groups. χ2 tests were used to compare ordinal/binary variables across groups.
bPerformance in the psychosis risk group did not significantly differ by ethnicity (ie, there was neither a main effect of ethnicity for overall accuracy on the PCLT, P = .65, nor for learning over time, P = .59; for striatal ROI fMRI analyses, P = .63).
cResults when excluding left-handed individuals were extremely similar for all analyses.
dResults when excluding medicated individuals were extremely similar for all analyses.
eSymptoms are rated on a 0–6 scale, ranging from “absent” to “severe and psychotic”; reported % having a rating of 3–5, signifying the presence of APS.
Participants in the comparison group (see table 1 for sample characteristics; n = 20) scored in the average range on both PerAb and MagicId scales (norms based on a large college student sample)33; in particular they scored greater than −0.58 SDs below the same sex mean and less than 0.53 SDs above the same sex mean on the PerAb and MagicId scales. In addition, the comparison group also scored less than 0.50 SD above the same sex mean on the Social Anhedonia Scale (SocAnh; M. Eckblad, L. Chapman, J. Chapman, M. Mishlove, unpublished data). In addition, comparison participants had to be rated less than 2 (2 = mild symptoms) on both the Unusual Thought Content/Delusional Ideation and the Perceptual Abnormalities/Hallucinations subscales of the SIPS. More information about materials used (eg, PerAb, MagicId, SocAnh, and SIPS) can be found in the supplementary methods.
Behavioral Tasks
Participants completed 2 tasks in the scanner in an alternating fashion: the Probabilistic Category Learning Task (PCLT) and a Perceptual-Motor Control Task (PMCT). On the PCLT,27,34,35 participants saw cards and predicted whether the card or combination of cards were associated with either rain or shine. The cards were composed of 4 simple geometric shapes. These 4 different shapes were either presented individually as a single card or in a group of up to 3 cards, with 14 different possible card combinations involving either an individual card or a group of cards. Each shape (and therefore each card combination) was associated with rain or shine on a probabilistic basis. Each trial began with the presentation of 1 of the 14 card combinations and participants gave their prediction by pressing 1 of 2 buttons on a response box. If the participant’s response was correct, the words “Correct!” and a yellow smiley face appeared; if incorrect, the words “Incorrect” and a red sad face appeared. Previous research has consistently found activation in the striatum on the PCLT, especially in the associative striatum.25–27
To examine whether the groups differed in task performance over the course of the task, behavioral data were analyzed using a group × trial multilevel logical regression for accuracy through the glmer procedure in R.36 Participants were modeled as random intercepts. It was expected that the comparison group, in contrast to the psychosis risk group, would exhibit significant improvement in task performance over the course of the task, as evidenced by positive accuracy slopes, indicating increased accuracy across trials. It was expected that the psychosis risk group would exhibit poorer learning across the task, as evidenced by a smaller slope than the comparison group. Furthermore, it was expected that groups would differ only on the last run of the task, as in a previous study examining psychosis risk using the PCLT,8 and therefore the groups were compared on each of the runs of the task (see supplementary methods and results for additional analyses regarding task strategy).
Following previous research,25,27 the PCLT was alternated with a control task (the PMCT) to control for neural activation associated with stimulus presentation and motor response. On each trial of the PMCT, participants decided whether 2 presented cards were identical or not. Following previous research,25,27 all stimuli (ie, for both the PCLT and the PMCT) were displayed on the screen for 4.5 s with an inter-trial interval of 0.5 s. Participants completed a total of 6 scan runs of these tasks. Each scan run was composed of 25 trials of the PCLT and 25 trials of the PMCT. During each scan run, there was a 29-s baseline fixation between the PCLT and PMCT runs, with an 11.5-s period of fixation at the beginning and end of the scan run (each fixation screen was composed of a blank screen with a fixation cross). Task order (PCLT first or PMCT first) was counterbalanced across groups, with no difference in PCLT accuracy between the 2 task orders, F(1, 39) = 0.00, P = .99, d = 0.00.
Imaging Data Analysis
Imaging took place at the Brain Imaging Center (BIC) at the University of Missouri with a 3T Siemens Trio scanner using an 8-channel head coil. Functional images were acquired for each participant using a T2*-weighted gradient-echo planar pulse sequence (4-mm slice thickness, TR = 2500, TE = 25, flip angle = 70, FOV = 256 mm × 256 mm). Following previous research,37,38 image slices were tilted 30° toward the coronal plane from the Anterior Commissure-Posterior Commissure (AC-PC) line to minimize artifact and improve scanning of the striatum. T1-weighted structural images were acquired for each participant using a high resolution T1-weighted sagittal scan (MPRAGE sequence, 176 sagittal slices, 1 mm slice thickness, TR = 1920 msec, TE = 2.93, flip angle = 9). FSL’s Brain Extraction Tool,39 was used to skull strip the structural images. Motion correction was performed using Motion Correction in FMRIB’s Linear Image Registration Tool (FLIRT).40 Structural images were cross-registered to a reference brain and images were smoothed (4-mm FWHM). Imaging data were analyzed using a mixed effects single-subject general linear model using FSL (http://www.fmrib.ox.ac.uk/fsl).41 Based on previous research,25,27 activation was analyzed in a blocked fashion (comparing PCLT activation with PMCT activation). Two covariates or explanatory variables were included: (a) PCLT activity and (b) PMCT activity. The groups were compared for regions that were both (a) significantly active during the PCLT and (b) significantly more active during the PCLT than the PMCT. Within-subject analyses examined the extent to which each voxel’s activity conformed to an a priori canonical double-gamma hemodynamic response function. In whole-brain analyses, cluster thresholding was used in which contiguous clusters were first identified with a threshold of Z = 2.30 and then used Gaussian random field theory to estimate each cluster’s estimated significance level, with significant clusters having a P < .05 FWE-corrected for multiple comparisons.40
To examine striatal activation on the PCLT, region of interest (ROI) analyses were first conducted. Activation in the striatum as a whole was examined first. Next, given that PCLT is especially related to activation of the associative striatum and that psychosis risk is especially related to associative striatum dysfunction, activation specifically in the associative striatum was examined. Our associative striatum ROI was created based on resting state analyses19 (our analyses aggregated the striatal subregions associated with the frontoparietal, default mode, and ventral attention networks; collectively, these subregions have been previously labeled as the associative striatum).21,42 To examine whether striatum activation was specific to the associative striatum, activation was also examined in 2 other striatal regions identified by Choi et al19 (eg, sensorimotor striatum and limbic striatum; ROIs were extracted from FWE-corrected analyses, and multiple comparisons were Bonferroni corrected). In addition to striatal ROI analyses, activation was also examined using whole-brain analyses, comparing the groups for regions that were both (a) significantly active during the PCLT and (b) significantly more active during the PCLT than the PMCT [see supplementary results for (a) correlations between PCLT accuracy with both striatal regions and regions with significant whole-brain group differences, and (b) correlations between symptoms with both striatal regions and PCLT accuracy].
Results
Behavioral Task Performance
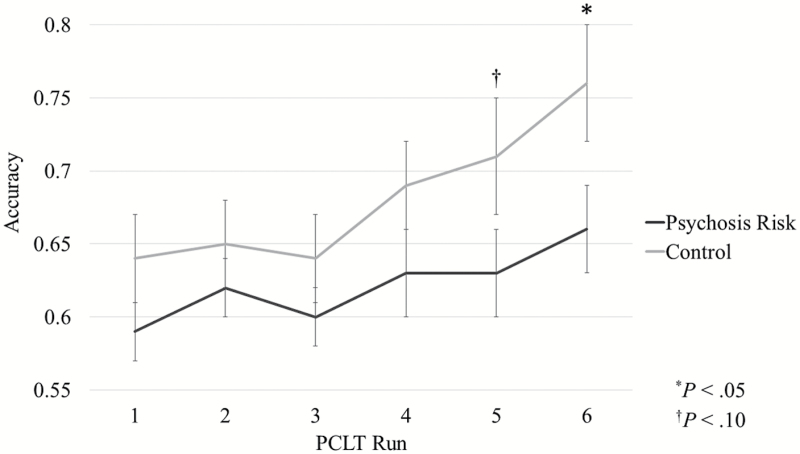
The groups were first compared on behavioral performance using a multilevel logistic regression analysis of accuracy over time (ie, across trials).8 As expected, there was a main effect of time, Z = 2.07, P < .05, d = 0.68, as accuracy on average improved over time (eg, for the comparison group, effect of time: Z = 4.46, P < .001, d = 1.25; for psychosis risk, effect of time: Z = 2.06, P = .04, d = 0.67). There was not an overall significant effect of group, Z = 0.57, P = .57, d = 0.18, which makes sense because this includes performance during the early, initial learning part of the task. However, as can be seen in figure 1, there was a trend toward a significant time × group interaction, Z = 1.72, P = .08, d = 0.56 (see supplementary table 1 for means and SDs). Furthermore, as expected, by the last run of the task the psychosis risk group did exhibit significantly lower accuracy than the comparison group, t(39) = 2.13, P < .05, d = 0.68, replicating previous results.8
Fig. 1.
Group comparisons of accuracy for each of the 6 runs on the Probabilistic Category Learning Task (PCLT) for psychosis risk and comparison groups. Error bars reflect standard errors (*P < .05, †P < .10).
Imaging Analyses
Striatal ROIs.
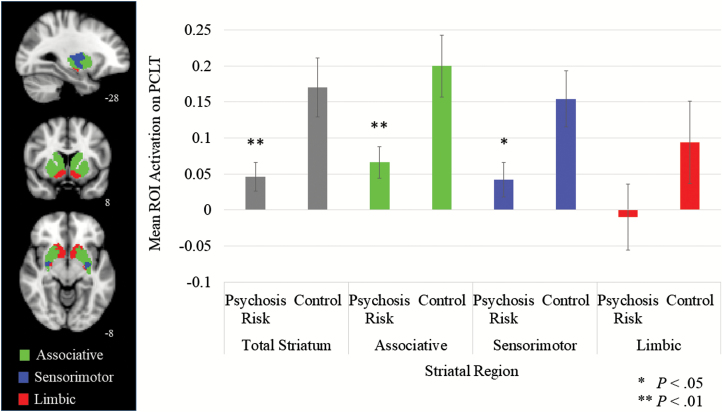
We next examined whether participants activated the striatum on the PCLT and whether the psychosis risk group exhibited decreased activation in the striatum. Across the entire sample, participants did exhibit increased overall striatal activity on the PCLT, t(39) = 4.40, P < .001, d = 1.41. Further, as expected and as can be seen in figure 2, the psychosis risk group exhibited significantly less overall striatal activation than the comparison group (P < .01, d = 0.86; see supplementary table 2 for means and SDs). Hence, the psychosis risk group exhibited both poorer behavioral performance on the PCLT as well as decreased striatal activation on this task.
Fig. 2.
Striatal region of interest (ROI) mean activation on the Probabilistic Category Learning Task (PCLT) for each group.
Next, analyses were conducted to examine whether performance on the PCLT was associated with activation in particular striatal regions and whether psychosis risk was associated with decreased activation in particular striatal regions. First of all, across the entire sample and as expected, there was significantly greater activation in the associative striatum on the PCLT than in either the sensorimotor or limbic striatal regions (Ps < .01, ds > .45, note that there was not a significant difference between the sensorimotor and limbic striatum, P > .05, d = 0.25). Further, as can be seen in figure 2, the psychosis risk group exhibited significantly decreased activation in the associative striatum than the comparison group, but not the sensorimotor or limbic striatum.
Whole-Brain fMRI Analyses.
Next, results for the whole-brain analyses on the PCLT were examined. As can be seen in supplementary table 3, consistent with previous research25,27,43 and the ROI analyses, several striatum/basal ganglia regions were identified as significantly activated on the PCLT. Further, just as for the ROI analyses, in whole-brain analyses the psychosis risk group also exhibited decreased striatal activation, specifically only within associative striatal regions.
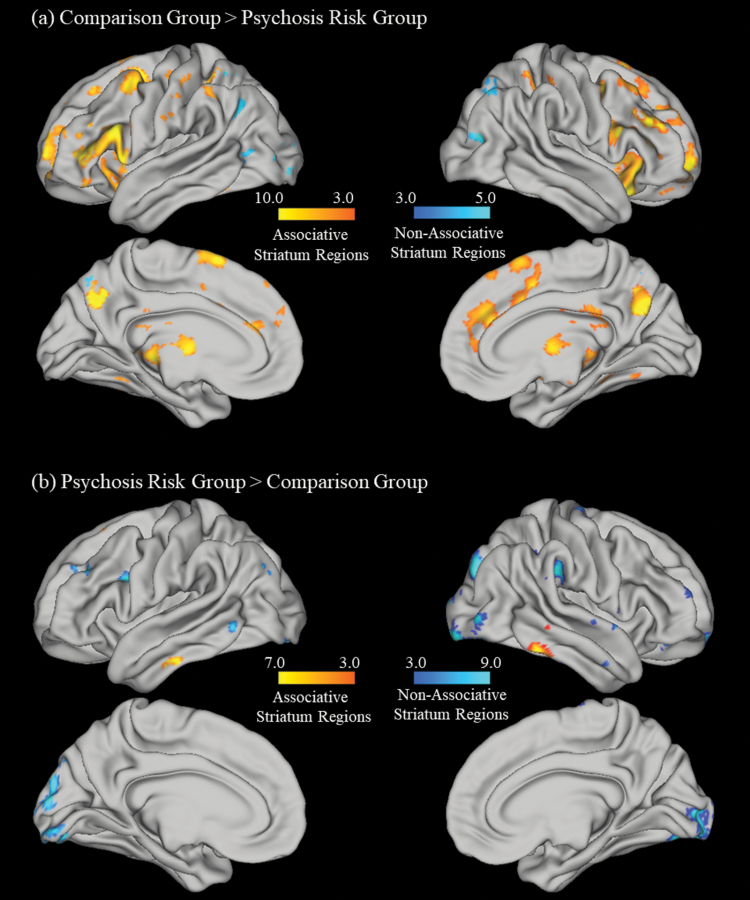
In terms of other brain regions significantly activated on the PCLT, overall across both groups, consistent with previous research and as can be seen in supplementary table 3, an extensive cluster of regions that included frontal, thalamic, insula, parietal, temporal, and occipital regions were significantly activated.27 As can be seen in figure 3 and table 2, the psychosis risk group also exhibited significantly less activity than the comparison group in multiple cortical regions connected to the associative striatum (ie, associative corticostriatal network regions; eg, several frontal, parietal, and temporal regions), as well as a superior lateral occipital cortex region associated with the occipital corticostriatal network.25,27 In contrast, as can be seen in figure 3 and table 2, psychosis risk participants had significantly greater PCLT activity in several regions than the comparison group, including several parietal and occipital regions not within the associative corticostriatal network (eg, the dorsal attention and occipital corticostriatal networks) as well one temporal region within the associative corticostriatal network.
Fig. 3.
Cortical regions where (a) comparison participants had significantly greater Probabilistic Category Learning Task (PCLT) activity than psychosis risk and (b) psychosis risk participants had significantly greater PCLT activity than comparison participants. Note the figures are divided into regions associated with the associative corticostriatal network (ie, “associative striatum regions,” shown in yellow) and regions not associated with the associative corticostriatal network (ie, “nonassociative striatum regions,” shown in blue).
Table 2.
Group Differences on the Probabilistic Category Learning Task in Whole-Brain Analyses
| Region | Volume of Voxels (mm3) | Peak Activity MNI Coordinates | Maximum Z-Statistic | Exact P-Value | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Regions significantly more active for the comparison group than psychosis risk | ||||||
| Right caudate | 197 | 12 | 14 | 8 | 3.78 | 0.0001 |
| Right putamen | 16 | 14 | −6 | 3.97 | 4.7e-05 | |
| Right hippocampus | 16 | −12 | −18 | 3.06 | 0.0012 | |
| Left thalamus | −4 | −18 | 6 | 4.11 | 2.7e-05 | |
| Right anterior cingulate cortex | 17855 | 10 | 44 | 8 | 4.48 | 5.7e-06 |
| Left inferior frontal gyrus | −46 | 38 | 8 | 6.59 | 1.3e-10 | |
| Right middle frontal gyrus | 48 | 34 | 24 | 10.5 | 6.0e-22 | |
| Left superior frontal gyrus | −22 | −2 | 58 | 4.97 | 6.4e-07 | |
| Right thalamus | 8 | −22 | 6 | 4.49 | 5.6e-06 | |
| Right superior parietal lobe | 2 | −70 | 38 | 6.22 | 1.1e-09 | |
| Regions significantly more active for psychosis risk than the comparison group | ||||||
| Right inferior temporal gyrus | 196 | 66 | −42 | −16 | 6.84 | 3.1e-11 |
| Right anterior intraparietal sulcus | 191 | 26 | −50 | 38 | 6.39 | 4.2e-10 |
| Right visual cortex (V4) | 757 | 32 | −78 | −18 | 9.72 | 2.1e-19 |
| Left visual cortex (V4) | 316 | −30 | −86 | −14 | 5.78 | 1.1e-08 |
Note: Analyses were also run with a cluster threshold of Z = 4.30, P > .01 FWE-corrected. For the analyses of regions significantly more active for the comparison group than psychosis risk, the results were largely similar, except that the hippocampus was no longer significantly activated. For the analyses of regions significantly more active for psychosis risk than the comparison group, there were no clusters of significant activation.
Discussion
The current study is to our knowledge the first to examine whether psychosis risk based on symptom levels is associated with both poor behavioral performance and impairments in specific striatal regions on the PCLT. The current research follows up previous work8 finding behavioral deficits in psychosis risk on the PCLT at the end of the task and finding novel evidence that psychosis risk is associated with striatum-related neural deficits associated with learning on this task. These results could help us better understand the nature of psychosis risk and also suggests that the PCLT could be a useful marker for psychosis risk.
In terms of their behavior, the psychosis risk group exhibited some evidence of impaired learning on the PLCT, as evidenced by reduced accuracy at the end of the task. This finding replicates previous research using this task to examine striatal-related behavioral impairments in psychosis risk,8 and is consistent with other research in individuals with increased genetic risk,7,10,12 elevated schizotypal traits,9 and schizophrenia.44,45 However, it should be noted that in contrast to previous research, there was not a significant effect of group on PCLT accuracy.9,10,27 Regardless, the current research suggests that category learning impairments may be a marker of risk for psychosis.
In terms of neural activation, the current study is to our knowledge the first study to find that psychosis risk based on symptom levels is associated with striatal dysfunction while performing the PCLT. Specifically, the psychosis risk group showed evidence, both in ROI and whole-brain analyses, of reduced associative striatum activation. These findings are consistent with previous PET, resting state, and functional fMRI research providing evidence that psychosis risk is associated with striatal dysfunction,5–7,12–16 especially within the associative striatum.4,20–23 The current study helps fill in the gaps of previous research by being the first study to indicate that striatum-related dysfunction can be detected in psychosis risk based on symptom levels using the PCLT.
Furthermore, there was evidence that psychosis risk was associated with dysfunction in cortical regions connected to the associative striatum. As previously mentioned, the striatum is critically involved in corticostriatal circuits,18,19 and parcellation research indicates distinguishable striatal regions.19 The cortical regions connected to the associative striatum include the prefrontal cortex (eg, inferior frontal gyrus, middle frontal gyrus), as well as parietal (eg, inferior parietal lobe, superior parietal lobe), and temporal (eg, hippocampal) regions. Results in the current study showed that when performing the PCLT, the comparison group activated cortical regions connected to the associative corticostriatal network (connected to the frontoparietal control, default mode, and ventral attention striatal subregions),19 in line with findings from comparison groups in previous studies using the PCLT.25,27 Furthermore, as expected, cortical activation within the associative corticostriatal network was significantly different between the psychosis risk and comparison groups, with the psychosis risk group showing significantly less activation in multiple cortical regions within this network, including reduced activation in the left inferior frontal gyrus and right middle frontal gyrus. Increased activation of the inferior temporal gyrus, anterior intraparietal sulcus, and visual regions in psychosis risk may be indicative of a compensatory mechanism that allowed the psychosis risk to exhibit some learning, albeit reduced, on this task. The current results are consistent with previous psychosis risk fMRI research finding reduced activation in some similar cortical regions when performing cognitively demanding tasks,15,46,47 including individuals at genetic risk while performing the PCLT.7 However, the current study is the first study to show psychosis risk based on symptom levels associated with decreased activation most consistently in regions within the associative corticostriatal network on the PCLT.
Overall, in the current study, the pattern of behavioral and neural activation is consistent with psychosis risk being associated with striatum-related impairments. The results also support an altered attribution of salience theory (as opposed to the classic aberrant salience theory).13,16,48 According to this theory, erratic phasic dopamine release, as is hypothesized in psychosis, may result in impaired prediction error processing, including deficits in the ability to use actual outcomes to drive goal-directed behavior (such as required for probabilistic category learning). Furthermore, previous research indicates that striatal dysfunction is associated with impairments using task feedback to improve performance, with increased striatal dopamine facilitating learning from positive feedback but impairing learning from negative feedback.49 Consistent with this, our previous research has found that psychosis risk is associated with impairments on a striatum-related neural response evoked by a reward and punishment-based learning task.50 Thus, striatal dopamine-mediated disruption in learning from feedback may result in the psychosis risk group showing greater impairments in category learning on the PCLT. To generalize these results to psychotic symptoms, continual impairments in feedback-mediated learning in the real-world over time may hypothetically lead to forming faulty generalized inferences from one’s experiences that could lead to the creation of delusional beliefs. Regardless of the interpretation, the psychosis risk group showed both behavioral and neural striatum-related impairments, consistent with the idea that striatum dysfunction is important for understanding psychosis risk. A strength of the current study is that the results are not confounded by antipsychotic medication as all participants were antipsychotic medication-naïve.
However, several limitations of the current study should be noted. First, a limitation of the study is the relatively small sample size (n = 20 per group). Second, another limitation is that the current study did not examine corticostriatal network functional connectivity. Lastly, the current study did not assess family history of schizophrenia, the presence of psychiatric diagnoses, recreational drug use, or IQ, which previous research indicates may be related to striatal functioning.10,51,52
This fMRI study is the first study to examine whether psychosis risk based on symptom levels is associated with dysfunction in specific striatal regions on the PCLT, finding that psychosis risk is associated with impaired associative striatal activation, as well as deficits in associative corticostriatal network regions. These results suggest that dysregulated associative corticostriatal functioning during category learning may predate the onset of psychotic disorders. Future studies should focus on continuing to understand the relationship between associative corticostriatal impairments in psychosis risk, including the relationship between corticostriatal connectivity and psychosis risk. The current study points to the PCLT as a potentially useful marker of psychosis risk and striatal dysfunction and suggests it could also be a useful marker in prevention treatment research of normalized striatal functioning and treatment success.53,54
Funding
This research was supported by the National Institute of Mental Health (T32 MH014677 to N.R.K. and MH100359 to J.G.K.) and by the University of Missouri research funds (to J.G.K.).
Supplementary Material
Acknowledgments
The authors declared no conflicts of interest in relation to the subject of this study.
References
- 1. Chapman LJ, Chapman JP, Kwapil TR, Eckblad M, Zinser MC. Putatively psychosis-prone subjects 10 years later. J Abnorm Psychol. 1994;103:171–183. [DOI] [PubMed] [Google Scholar]
- 2. Pedersen CB, Mors O, Bertelsen A, et al. . A comprehensive nationwide study of the incidence rate and lifetime risk for treated mental disorders. JAMA Psychiatry. 2014;71:573–581. [DOI] [PubMed] [Google Scholar]
- 3. Faridi K, Pawliuk N, King S, Joober R, Malla AK. Prevalence of psychotic and non-psychotic disorders in relatives of patients with a first episode psychosis. Schizophr Res. 2009;114:57–63. [DOI] [PubMed] [Google Scholar]
- 4. Fusar-Poli P, Howes OD, Allen P, et al. . Abnormal prefrontal activation directly related to pre-synaptic striatal dopamine dysfunction in people at clinical high risk for psychosis. Mol Psychiatry. 2011;16:67–75. [DOI] [PubMed] [Google Scholar]
- 5. Juckel G, Friedel E, Koslowski M, et al. . Ventral striatal activation during reward processing in subjects with ultra-high risk for schizophrenia. Neuropsychobiology. 2012;66:50–56. [DOI] [PubMed] [Google Scholar]
- 6. Rausch F, Mier D, Eifler S, et al. . Reduced activation in the ventral striatum during probabilistic decision-making in patients in an at-risk mental state. J Psychiatry Neurosci. 2015;40:163–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wagshal D, Knowlton BJ, Suthana NA, et al. . Evidence for corticostriatal dysfunction during cognitive skill learning in adolescent siblings of patients with childhood-onset schizophrenia. Schizophr Bull. 2014;40:1030–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Karcher NR, Martin EA, Kerns JG. Examining associations between psychosis risk, social anhedonia, and performance of striatum-related behavioral tasks. J Abnorm Psychol. 2015;124:507–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Skilleter AJ, Weickert CS, Moustafa AA, et al. . BDNF val66met genotype and schizotypal personality traits interact to influence probabilistic association learning. Behav Brain Res. 2014;274:137–142. [DOI] [PubMed] [Google Scholar]
- 10. Wagshal D, Knowlton BJ, Cohen JR, et al. . Impaired automatization of a cognitive skill in first-degree relatives of patients with schizophrenia. Psychiatry Res. 2014;215:294–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Waltz JA, Demro C, Schiffman J, et al. . Reinforcement learning performance and risk for psychosis in youth. J Nerv Ment Dis. 2015;203:919–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Weickert TW, Goldberg TE, Egan MF, et al. . Relative risk of probabilistic category learning deficits in patients with schizophrenia and their siblings. Biol Psychiatry. 2010;67:948–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Radua J, Schmidt A, Borgwardt S, et al. . Ventral striatal activation during reward processing in psychosis: a neurofunctional meta-analysis. JAMA Psychiatry. 2015;72:1243–1251. [DOI] [PubMed] [Google Scholar]
- 14. Roiser JP, Howes OD, Chaddock CA, Joyce EM, McGuire P. Neural and behavioral correlates of aberrant salience in individuals at risk for psychosis. Schizophr Bull. 2013;39:1328–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schmidt A, Antoniades M, Allen P, et al. . Longitudinal alterations in motivational salience processing in ultra-high- risk subjects for psychosis. Psychol Med. 2017;47:243–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wotruba D, Heekeren K, Michels L, et al. . Symptom dimensions are associated with reward processing in unmedicated persons at risk for psychosis. Front Behav Neurosci. 2014;8:382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gerfen CR, Surmeier DJ. Modulation of striatal projection systems by dopamine. Annu Rev Neurosci. 2011;34:441–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Alexander GE, Crutcher MD, DeLong MR. Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Prog Brain Res. 1990;85:119–146. [PubMed] [Google Scholar]
- 19. Choi EY, Yeo BT, Buckner RL. The organization of the human striatum estimated by intrinsic functional connectivity. J Neurophysiol. 2012;108:2242–2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Egerton A, Chaddock CA, Winton-Brown TT, et al. . Presynaptic striatal dopamine dysfunction in people at ultra-high risk for psychosis: findings in a second cohort. Biol Psychiatry. 2013;74:106–112. [DOI] [PubMed] [Google Scholar]
- 21. Howes OD, Montgomery AJ, Asselin MC, et al. . Elevated striatal dopamine function linked to prodromal signs of schizophrenia. Arch Gen Psychiatry. 2009;66:13–20. [DOI] [PubMed] [Google Scholar]
- 22. Stone JM, Howes OD, Egerton A, et al. . Altered relationship between hippocampal glutamate levels and striatal dopamine function in subjects at ultra high risk of psychosis. Biol Psychiatry. 2010;68:599–602. [DOI] [PubMed] [Google Scholar]
- 23. Dandash O, Fornito A, Lee J, et al. . Altered striatal functional connectivity in subjects with an at-risk mental state for psychosis. Schizophr Bull. 2014;40:904–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fornito A, Harrison BJ, Goodby E, et al. . Functional dysconnectivity of corticostriatal circuitry as a risk phenotype for psychosis. JAMA Psychiatry. 2013;70:1143–1151. [DOI] [PubMed] [Google Scholar]
- 25. Fera F, Weickert TW, Goldberg TE, et al. . Neural mechanisms underlying probabilistic category learning in normal aging. J Neurosci. 2005;25:11340–11348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Poldrack RA, Clark J, Paré-Blagoev EJ, et al. . Interactive memory systems in the human brain. Nature. 2001;414:546–550. [DOI] [PubMed] [Google Scholar]
- 27. Weickert TW, Goldberg TE, Callicott JH, et al. . Neural correlates of probabilistic category learning in patients with schizophrenia. J Neurosci. 2009;29:1244–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cicero DC, Martin EA, Becker TM, Docherty AR, Kerns JG. Correspondence between psychometric and clinical high risk for psychosis in an undergraduate population. Psychol Assess. 2014;26:901–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chapman LJ, Chapman JP, Raulin ML. Body-image aberration in schizophrenia. J Abnorm Psychol. 1978;87:399–407. [DOI] [PubMed] [Google Scholar]
- 30. Eckblad M, Chapman LJ. Magical ideation as an indicator of schizotypy. J Consult Clin Psychol. 1983;51:215–225. [DOI] [PubMed] [Google Scholar]
- 31. Miller TJ, McGlashan TH, Rosen JL, et al. . Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr Bull. 2003;29:703–715. [DOI] [PubMed] [Google Scholar]
- 32. Debbané M, Eliez S, Badoud D, Conus P, Flückiger R, Schultze-Lutter F. Developing psychosis and its risk states through the lens of schizotypy. Schizophr Bull. 2015;41(suppl 2):S396–S407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kerns JG, Berenbaum H. Aberrant semantic and affective processing in people at risk for psychosis. J Abnorm Psychol. 2000;109:728–732. [DOI] [PubMed] [Google Scholar]
- 34. Knowlton BJ, Squire LR, Gluck MA. Probabilistic classification learning in amnesia. Learn Mem. 1994;1:106–120. [PubMed] [Google Scholar]
- 35. Wagshal D, Knowlton BJ, Cohen JR, et al. . Cognitive correlates of gray matter abnormalities in adolescent siblings of patients with childhood-onset schizophrenia. Schizophr Res. 2015;161:345–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ihaka R, Gentleman R. R: a language for data analysis and graphics. Journal of Comput Graph Stat. 1996;5:299–314. [Google Scholar]
- 37. Alexander WH, Brown JW. Competition between learned reward and error outcome predictions in anterior cingulate cortex. Neuroimage. 2010;49:3210–3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Deichmann R, Gottfried JA, Hutton C, Turner R. Optimized EPI for fMRI studies of the orbitofrontal cortex. Neuroimage. 2003;19(2 Pt 1):430–441. [DOI] [PubMed] [Google Scholar]
- 39. Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. [DOI] [PubMed] [Google Scholar]
- 41. Smith SM, Jenkinson M, Woolrich MW, et al. . Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(suppl 1):S208–S219. [DOI] [PubMed] [Google Scholar]
- 42. Kegeles LS, Abi-Dargham A, Frankle WG, et al. . Increased synaptic dopamine function in associative regions of the striatum in schizophrenia. Arch Gen Psychiatry. 2010;67: 231–239. [DOI] [PubMed] [Google Scholar]
- 43. Celone KA, Thompson-Brenner H, Ross RS, Pratt EM, Stern CE. An fMRI investigation of the fronto-striatal learning system in women who exhibit eating disorder behaviors. Neuroimage. 2011;56:1749–1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Horan WP, Green MF, Knowlton BJ, Wynn JK, Mintz J, Nuechterlein KH. Impaired implicit learning in schizophrenia. Neuropsychology. 2008;22:606–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Weickert TW. Probabilistic association learning in schizophrenia. Curr Opin Behav Sci. 2018;20:1–8. [Google Scholar]
- 46. Colibazzi T, Horga G, Wang Z, et al. . Neural dysfunction in cognitive control circuits in persons at clinical high-risk for psychosis. Neuropsychopharmacology. 2016;41:1241–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fusar-Poli P, Howes OD, Allen P, et al. . Abnormal frontostriatal interactions in people with prodromal signs of psychosis: a multimodal imaging study. Arch Gen Psychiatry. 2010;67:683–691. [DOI] [PubMed] [Google Scholar]
- 48. Heinz A, Schlagenhauf F. Dopaminergic dysfunction in schizophrenia: salience attribution revisited. Schizophr Bull. 2010;36:472–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Frank MJ, Seeberger LC, O’reilly RC. By carrot or by stick: cognitive reinforcement learning in Parkinsonism. Science. 2004;306:1940–1943. [DOI] [PubMed] [Google Scholar]
- 50. Karcher NR, Bartholow BD, Martin EA, Kerns JG. Associations between electrophysiological evidence of reward and punishment-based learning and psychotic experiences and social anhedonia in at-risk groups. Neuropsychopharmacology. 2017;42:925–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chen C. Intelligence moderates reinforcement learning: a mini-review of the neural evidence. J Neurophysiol. 2015;113:3459–3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Martz ME, Trucco EM, Cope LM, et al. . Association of Marijuana use with blunted nucleus accumbens response to reward anticipation. JAMA Psychiatry. 2016;73:838–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Minzenberg MJ, Carter CS. Developing treatments for impaired cognition in schizophrenia. Trends Cogn Sci. 2012;16:35–42. [DOI] [PubMed] [Google Scholar]
- 54. Sommer IE, Bearden CE, van Dellen E, et al. . Early interventions in risk groups for schizophrenia: what are we waiting for?NPJ Schizophr. 2016;2:16003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.