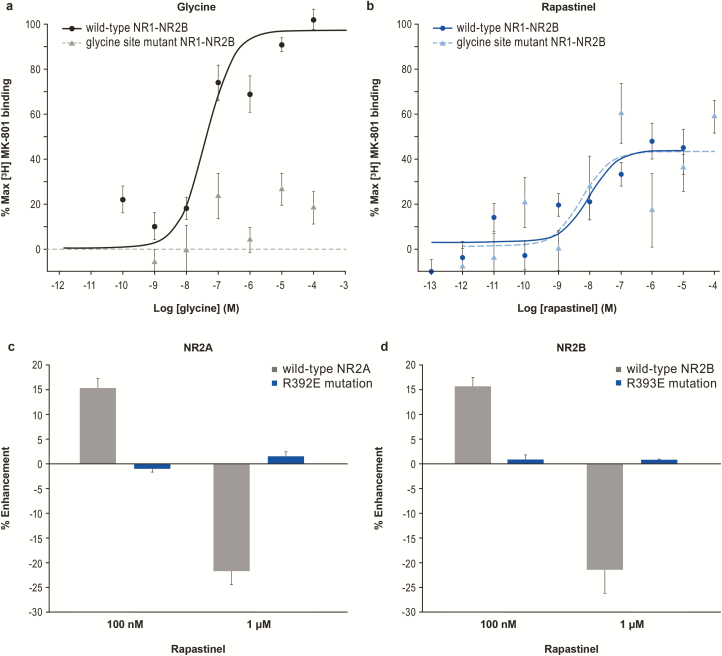
Figure 5.
Rapastinel functional site is different from the glycine binding site. (A) Glycine increased [3H] MK-801 binding in wild-type NR1-NR2B controls but not in NR1-NR2B-expressing HEK cells containing a loss of function mutation in the glycine binding site (F484A/T518L) (n = 18). (B) Rapastinel increased [3H] MK-801 binding in both wild-type and glycine mutant cells (n = 18, 2-tailed t test). The modulation of N-methyl-D-aspartate receptor (NMDA)-induced intracellular calcium response of rapastinel was abolished in HEK293 cells transiently transfected with a (C) R392E mutation in NR2A and (D) R393E mutation in NR2B. Data are mean ± SEM.