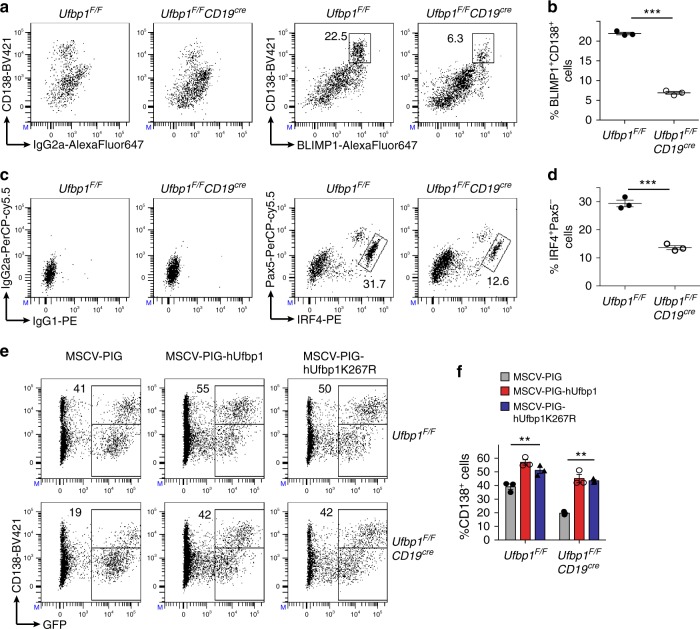
Fig. 3.
Defective differentiation of Ufbp1-deficient B cells into plasmablasts. a, c Sorted naive B cells from lymph nodes of indicated mice were cultured with lipopolysaccharide (LPS; 1 µg/ml) + interleukin (IL)-4 (10 ng/ml) + IL-5 (5 ng/ml). Four days later cells were analyzed for expression of BLIMP1, CD138, IRF4, and Pax5 by flow cytometry. Left panels in a and c show control IgG2a, IgG1, and IG2a isotype staining for BLIMP1, IRF4, and Pax5 respectively. Right panels in a and c show staining for BLIMP1 and CD138, and IRF4 and Pax5 respectively. Numbers represent the percent positive cells in the adjacent/corresponding area. b Enumeration of the frequency of BLIMP1+CD138high cells in a (n = 3 mice/genotype). d Enumeration of the frequency of IRF4+Pax5− cells in c (n = 3 mice/genotype). e Naive B cells from Ufbp1F/F and Ufbp1F/FCD19cre mice were cultured with LPS, IL-4, and IL-5 as above, and transduced with retrovirus containing empty vector (MSCV-PIG), expressing human Ufbp1 (MSCV-PIG-hUfbp1) or Ufbp1K267R (MSCV-PIG-hUfbp1K267R). Transduction of cells with retroviruses was monitored by expression of green fuorescent protein (GFP). Two days after transduction, cells were analyzed for GFP and CD138. Numbers represent percent CD138+ cells within GFP+ cells. f Enumeration of the frequency of CD138+ cells among GFP+ cells in e (n = 3 mice/genotype). Error bars represent mean ± standard error. **P < 0.01, ***P < 0.001. Unpaired Student’s two-tailed t-test was used. A representative of at least two experiments is shown