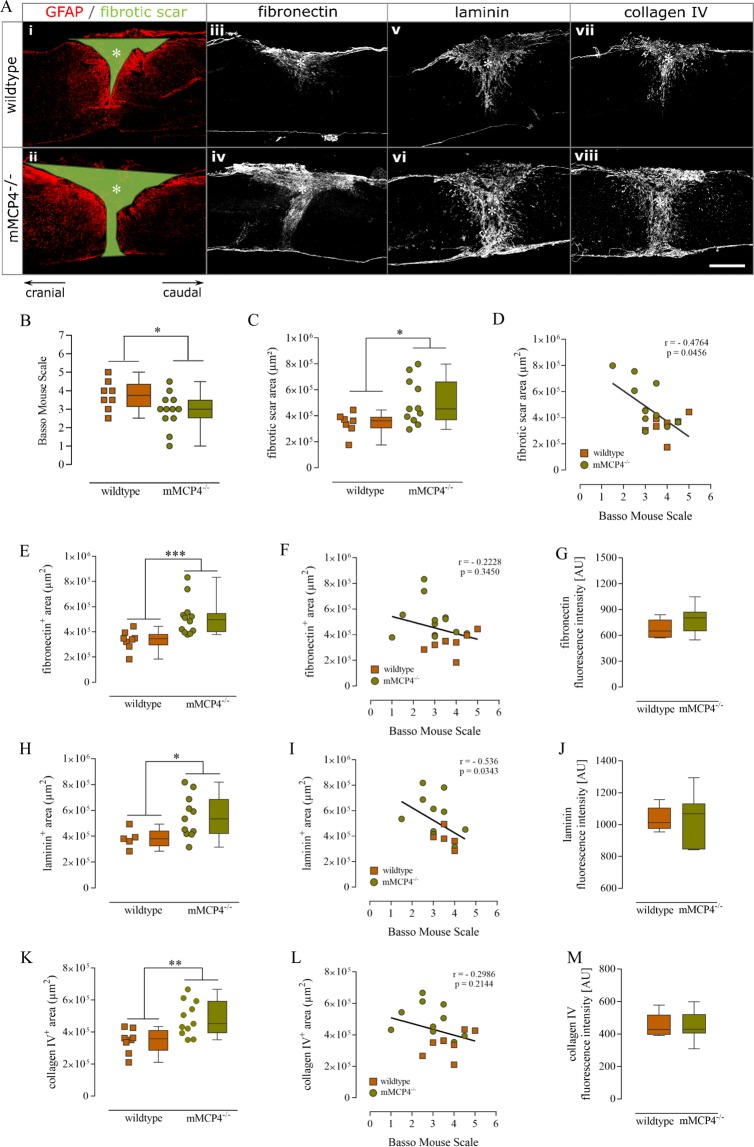
Figure 1.
Impaired functional recovery and increased fibrotic scar formation in mMCP4 knockout mice after SCI. (A) Representative fluorescent photomicrographs of the fibrotic scar at the lesion site in WT and mMCP4−/− mice, respectively. Scale bar in all images = 500 µm. GFAP is visualized in red in Ai/ii. (B) The deficiency of mMCP4 in mMCP4 knockout mice results in a significantly decreased BMS score after SCI. WT mice: n = 8; mMCP4−/− mice: n = 12. (C) A significant increase in fibrotic scar area (i.e. GFAP negative area marked in green in Ai/ii) was observed in mMCP4 knockout mice (Aii) compared to WT controls (Ai) at 28 dpi. (D) Inverse correlation between fibrotic scar formation and functional outcome after SCI in our mouse model (p = 0.0456, r = −0.4764, Spearman rank correlation coefficient). (E,H,K) To characterize the fibrotic scar response in more detail, we examined the expression of fibronectin and of the basement membrane components laminin and collagen IV. We found that the fibronectin- (E), laminin- (H) and collagen IV-positive (K) area were significantly increased in mMCP4−/− mice (Aiv/vi/viii) compared to WT mice (Aiii/v/vii). (F,I,L) A significant inverse correlation was observed between the laminin-positive area and the functional outcome (p = 0.0343, r = −0.536) (I), but not with the other matrix components (F,L). (G,J,M) In contrast to the area, the intensity of the immunoreactivity of these extracellular matrix components at the lesion was comparable between WT and mMCP4−/− mice. Individual data points are shown per mouse, together with the corresponding boxplots with the median and whiskers indicating the minimum and maximum. Histological analyses were performed on 5–8 WT mice and 10-12 mMCP4−/− mice. AU: arbitrary units. Asterisks in the fluorescent images indicate the lesion center. *p < 0.05; **p < 0.01; ***p < 0.001.