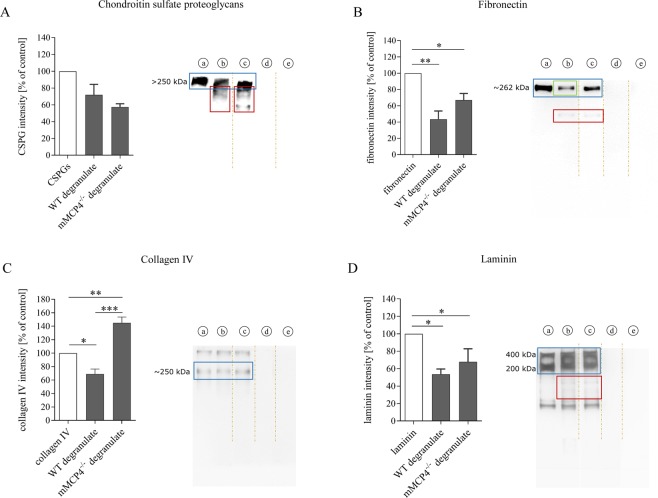
Figure 3.
mMCP4 degrades scar-associated ECM components in vitro. (A–D) Recombinant fibronectin, collagen IV, laminin or a CSPG-mix (aggrecan, neurocan, phosphacan, versican) were incubated with degranulate from WT or mMCP4−/− MCs to measure protein degradation. (A) Fragments of CSPG degradation products are visible at lower molecular weight levels after incubation with degranulate from WT or mMCP4−/− MCs (red-boxed areas in A). No statistically significant difference in CSPG intensity was observed between the groups. (B) Fibronectin was cleaved by degranulate from both WT and mMCP4−/− MCs (the red-boxed area indicates cleavage fragments); although with reduced cleavage (trend) by degranulate from mMCP4−/− vs. WT MCs (the green box indicates a stronger reduction in intensity of the fibronectin protein band after incubation with MCWT degranulate). (C) Collagen IV was cleaved by degranulate from WT MCs, but not degranulate from mMCP4−/− MCs. (D) Laminin was cleaved by degranulate from both WT and mMCP4−/− MCs (the red box indicates cleavage fragments). Data were normalized to the control condition (a) and presented as mean ± SEM; n = 3-4 experimental repeats/condition; *p < 0.05, **p < 0.01, ***p < 0.001. Legend: (a) = recombinant protein; (b) = recombinant protein + MCWT degranulate; (c) = recombinant protein + MCmMCP4−/− degranulate; (d) = degranulate from WT MCs alone; (e) degranulate from mMCP4−/− MCs alone. Intensity analysis were performed on the main protein bands that correspond with the known molecular weights of the ECM components (blue boxes). Brown dotted lines in the images indicate that the original blots have been cropped to exclude data on mMCP6 degradation which are not the focus of this study and have been published in Vangansewinkel et al.34. Original uncropped Western blot images are shown in Supplementary Fig. S1.