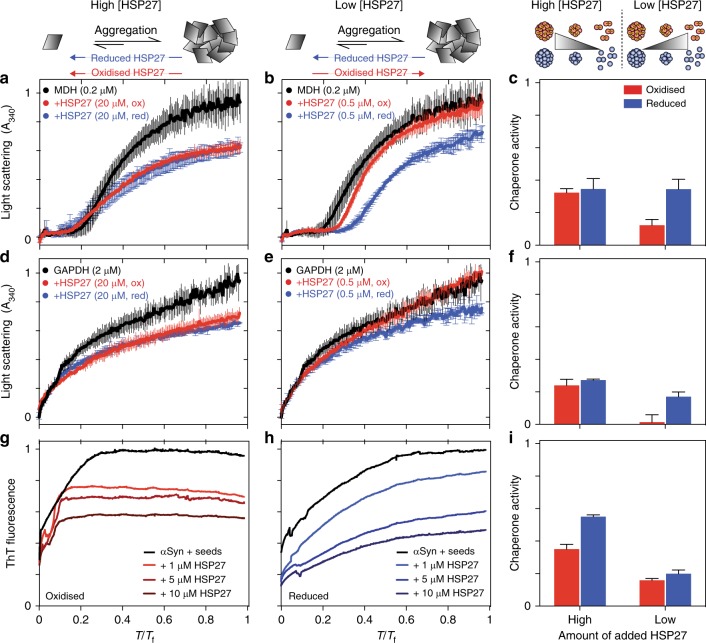
Fig. 2.
HSP27 monomers are potent chaperones in vitro. The aggregation of MDH (a, b) and GAPDH (d, e) was monitored by light scattering at 340 nm and seeded amyloid fibril formation by αS (g, h) was monitored by ThT fluorescence. Reduced (blue) or oxidised (red) HSP27 was added at either high (≥10 μM) concentrations (a, d, g) where it is predominantly oligomeric or low (≤1 μM) concentrations (b, e, h) where a large fraction of dimers (oxidised) or monomers (reduced) are populated. The y axes were normalised for comparison, and the x axes were scaled by a factor Tf to normalise time. The Tf values used for each substrate were 5 (a, b), 5 (d, e), and 170 (g, h). The chaperone activity of HSP27 against each substrate (c, f, i) is quantified as one minus the ratio of average signal over the time course with and without chaperone (Methods). Values of one and zero would respectively represent the complete inhibition of aggregation and no protection against aggregation. The average of three replicates is shown with error bars corresponding to ±1 SD