Abstract
INTRODUCTION: Gender-specific differences have led to the androgen receptor (AR) being considered a possible factor in the pathophysiology of urothelial carcinoma of the bladder (UCB), but the exact role remains unclear. MATERIALS AND METHODS: The association of AR mRNA expression with clinicopathological features was retrospectively analyzed in two previously described cohorts. The first cohort consisted of 41 patients with all stages of UCB treated at Aarhus University Hospital, Denmark. The second cohort consisted of 323 patients with muscle-invasive bladder cancer (MIBC) accumulated by the Cancer Genome Atlas (TCGA) Research Network. RESULTS: AR mRNA expression is significantly higher in non-muscle-invasive bladder cancer (NMIBC) when compared to MIBC (P = .0004), with no relevant changes within the different stages of MIBC. AR mRNA expression was significantly associated with TCGA molecular subtypes (P < .0001). In the total cohort, there was no association between AR expression and gender (P = .23). When analyzed separately, females showed a significantly worse disease-free (P = .03) and overall survival (P = .02) when expressing AR mRNA above median level, while the same was not observed for men. Multivariable Cox's regression analyses revealed AR mRNA expression to be an independent prognostic marker for disease-free survival in women (P = .007). CONCLUSIONS: AR mRNA expression is significantly higher in NMIBC than in MIBC, while high AR mRNA expression is associated with worse survival in females with MIBC. Further studies need to investigate the gender-specific role of AR in UCB.
Introduction
Urothelial carcinoma of the bladder (UCB) is the ninth most common cancer worldwide with an estimated 429,000 new cases and 165,000 deaths per year [1]. Men have a three to four times increased risk of developing bladder cancer than women, even when accounting for lifestyle and environmental factors [2], [3]. On the other hand, women are more likely to have advanced tumors at the time of diagnosis as well as a worse outcome [4], [5]. This has led to the investigation of sex hormones and sex hormone receptors as possible factors in the development of bladder cancer [6].
There is emerging evidence of the involvement of the androgen receptor (AR) and the androgen signaling pathway in urothelial carcinoma [7]. An association between expression of AR and the development of UCB could be demonstrated in numerous experiments on animals and human urothelial cell lines [8], [9], [10], [11], [12]. However, the prognostic relevance of AR expression in UCB remains controversial. While some studies described an association between high AR expression and increased tumor stage and grade as well as a worse survival [8], [13], other studies reported that tumor progression is accompanied by decreased AR expression [14], [15]. Moreover, most studies found no significant difference of AR expression in UCB between male and female patients [13], [15], [16]. The conflicting results of the previously cited studies might in part be attributed to immunohistochemistry as the main method to determine AR expression, since immunohistochemistry is associated with high inter-observer variability depending on the antibodies used and different cut-off values [16]. In a recent study, we were able to identify improved survival for patients with stage T1 bladder cancer and high AR mRNA expression measured with quantitative real-time polymerase chain reaction [17].
Additionally, an association between AR and the transcription factor GATA3 was previously investigated in UCB by analogy with breast cancer. Prior studies in breast cancer demonstrated that high co-expression of GATA3 and AR was a key feature of the apocrine subtype of triple negative breast cancers, which is associated with improved prognosis compared to other types of triple negative breast cancers [18]. In UCB, previous preclinical studies in UCB cell lines showed that GATA3 knockdown results in down-regulation of molecules that play a protective role in bladder tumorigenesis and up-regulation of oncogenic genes, thus suggesting a protective role of GATA3 in bladder cancer [19]. Moreover, GATA3 is considered one of the main markers to characterize luminal subtypes of UCB [20], [21]. A prognostic significance of GATA3 in UCB as well as a possible regulatory interaction with androgens has previously also been suggested [19], [22], but further analyses are still necessary.
In the present study, we retrospectively investigated the association of AR mRNA expression with clinicopathological features, survival, and GATA3 mRNA expression within two independent cohorts representing patients with all stages of UCB.
Materials and Methods
Patient Population
In the present study we were given access to two previously described cohorts for the analysis of AR mRNA expression.
The first cohort consisted of 41 patients with all stages of UCB (Ta – T4) at initial diagnosis originally treated by transurethral resection of the bladder (TURB) at the Department of Urology of the Aarhus University Hospital. Informed consent was obtained from all patients, and the protocols were approved by the scientific ethical committee of Aarhus County and performed in accordance to corresponding approved regulations and guidelines [23]. Samples were obtained directly from surgery and frozen in a guanidinium thiocyanate solution for preservation of RNA. Only specimens containing at least 50% tumor cells were used in this study which was previously suggested to be valid for mRNA based subtyping in breast cancer studies [24], [25], [26]. A full-genome expression analysis of 59,619 genes and expressed sequence tags including sex hormone receptor genes was performed by high-density oligonucleotide microarrays (customized Affymetrix GeneChip, Santa Clara, CA) as previously described in more detail [23]. Data on tumor stage, tumor grade according to the 1973 WHO classification and AR mRNA expression was available for all patients. There were no data on age and treatment modality and only limited data on nodal and metastasis status. All patients of this cohort were included in the analysis of AR mRNA expression with tumor stage and grade.
The second cohort comprised 402 cases that were accumulated by the Cancer Genome Atlas (TCGA) Research Network from 19 sites [27]. RNA-Seq (HiSeq) was used for whole genome analysis in tumor samples as previously described [27]. Four molecular subtypes were defined in this cohort based on mRNA expression patterns (i.e. TCGA subtypes): the subtypes I and II are described as luminal-like, with subtype I being defined by FGFR3 alterations and elevated FGFR3 expression, while subtype II is characterized by ERBB2 mutations and estrogen receptor beta (ESR2) enrichment. Subtypes III and IV are described as basal-like, defined by increased expression of epithelial lineage genes and stem/progenitor cytokeratines, with subtype III showing higher mRNA expression of keratins and FGFR3 than subtype IV [27].
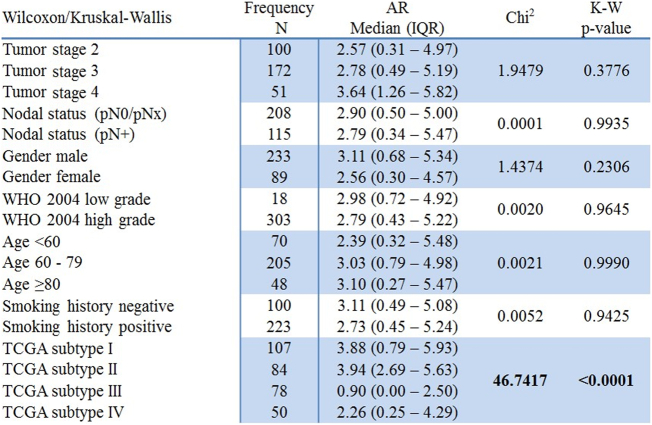
The clinical data as well as data on AR and GATA3 mRNA expression for the TCGA cohort is publicly available at the cBioPortal for Cancer Genomics website (http://www.cbioportal.org/study?id=blca_tcga#clinical). The data set was downloaded on December 6th of 2016 and was validated by K.A.H. on March 16th of 2017. Since most patients in the TCGA cohort had MIBC, patients with stages T0 and T1 were excluded from the analysis. Aside from 10 patients who received radiotherapy, the exact treatment modality was not documented. Therefore, we used a documented pN status as a surrogate for surgical therapy. All patients with pNX were excluded from the analysis, as were patients who received neoadjuvant therapy, definitive radiotherapy or had documented metastases. The final analysis set consisted of 323 patients. We then analyzed the association of AR mRNA expression in MIBC with tumor stage, tumor grade according to the WHO 2004 classification, nodal status, age, gender, smoking status, TCGA subtype as well as overall (OS) and disease-free survival (DFS). We also decided to analyze the association between AR and GATA3 mRNA expression, since an association was previously suggested [19], [22].
Statistical Methods
The Spearman's product–moment correlation coefficient rs was used to measure the strength and direction of the linear relationship between variables (tumor stage, grade, nodal status, gender, age, smoking status, GATA3 expression and TCGA subtype). In addition, Wilcoxon/Kruskal-Wallis was used to test the significance of the differences between these variables. Scatter plot and box plot analysis were used to describe AR mRNA expression depending on stage and TCGA subtype. Statistical analysis including Kaplan–Meier survival analysis and multivariable Cox's regression analysis were performed with JMP SAS (SAS Institute, Cary, NC, USA) and Graph Pad Prism software (Version 5.04; Graph Pad Software Inc., La Jolla, CA, USA).
Results
Results from the Aarhus Cohort
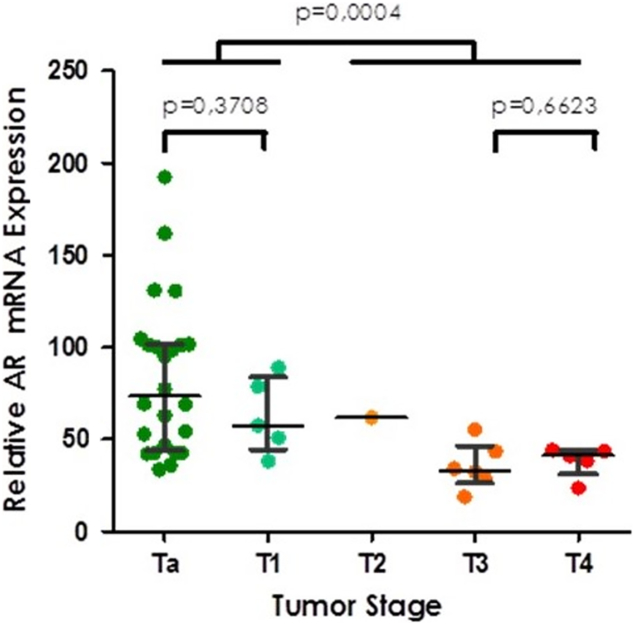
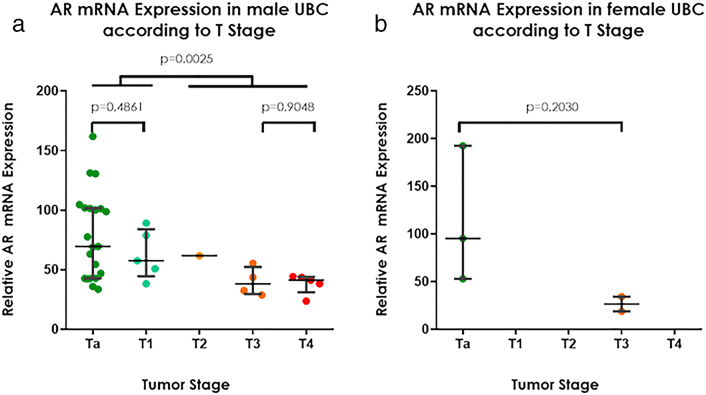
Clinicopathological characteristics of the Aarhus cohort are summarized in Table 1. Of the 41 patients, 29 had non-muscle-invasive bladder cancer (NMIBC) and 12 had muscle-invasive bladder cancer (MIBC). The median AR mRNA expression in this cohort was 54.4. Since there were no data on treatment modality and only limited data on nodal and distant metastases, we did not analyze the association between AR mRNA expression and nodal status, metastasis and survival. Spearman's rank correlation and Wilcoxon/Kruskal-Wallis testing revealed a statistically significant association between tumor stage (Chi2 = 14.48; P = .0059) but not tumor grade (Chi2 = −0.53; P = .46). Scatter plot analysis demonstrated a significantly lower AR mRNA expression pattern with increasing tumor stage, with the largest gap occurring between NMIBC and MIBC (P = .0004) (Figure 1). There was no association between AR mRNA expression and gender in the complete cohort. Median mRNA expression in females was almost identical to males (52.93 vs 54.82) with similar range (Min/Max females 18.84–192.45 vs Min/Max males 23.67–161.84). The analysis of stage dependent AR mRNA expression stratified by gender showed a trend towards higher AR mRNA expression in NMIBC, however, because of the small sample size containing only five women the gender specific analysis could not reveal any statistically meaningful results (Supplementary Figure 1).
Table 1.
Patient characteristics of the patients in the Aarhus cohort
| Patient characteristics | n (%) |
|---|---|
| Total cohort | 41 (100) |
| Gender | |
| male | 36 (88) |
| female | 5 (12) |
| Tumor stage | |
| Ta | 24 (58.5) |
| T1 | 5 (12.2) |
| T2 | 1 (2.4) |
| T3 | 6 (14.6) |
| T4 | 5 (12.2) |
| Nodal status | |
| N+ | 5 (12.2) |
| N0 | 2 (4.9) |
| Nx | 34 (82.9) |
| Metastasis stage | |
| M+ | 6 (14.6) |
| M0 | 6 (14.6) |
| Mx | 29 (70.7) |
| Tumor grade (WHO 1973) | |
| G1 | 0 (0) |
| G2 | 10 (24.4) |
| G3 | 31 (75.6) |
Figure 1.
AR mRNA expression in the Aarhus cohort according to pathological tumor stage.
Supplementary Figure 1.
AR mRNA expression in the Aarhus cohort according to pathological tumour stage in men (a) and women (b).
Results from the TCGA Cohort
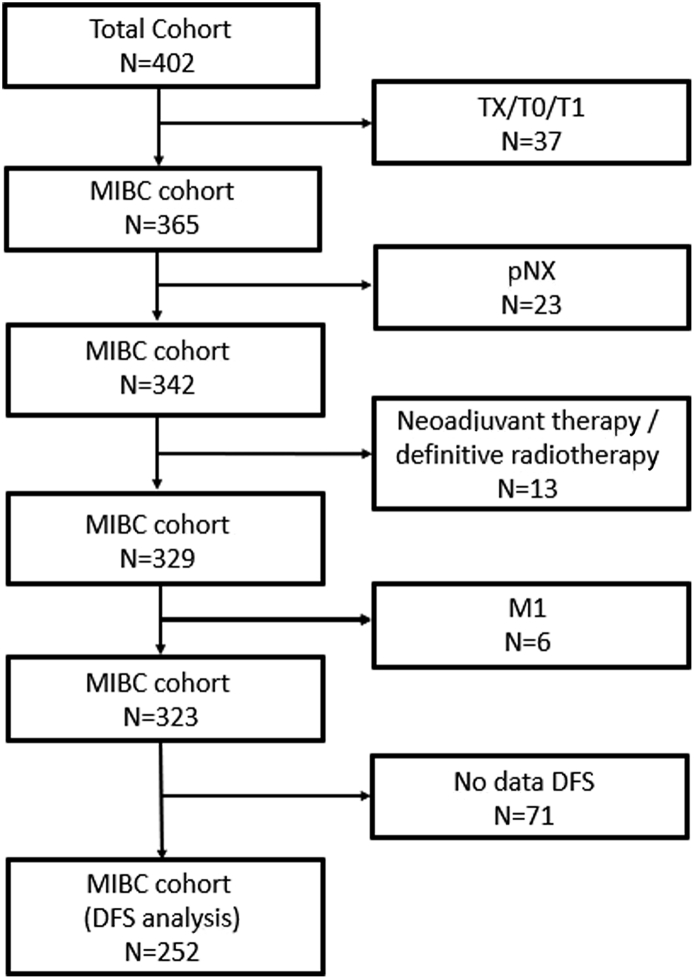
A total of 79 patients were excluded (4 patients had NMIBC; 33 had no data on tumor stage; 41 had no data on nodal status; 10 had positive metastases; 10 received neoadjuvant therapy; 10 received definitive radiotherapy; some patients had more than one exclusion criteria), leaving 323 patients with MIBC to be included into the AR mRNA expression analysis. Figure 2 shows the flow chart representing the exclusion criteria. An additional 71 patients were excluded from the analysis of DFS because of missing data, leaving a total of 252 patients (183 male (72.6%) and 69 female (27.4%)) for inclusion in the analysis of the association of AR mRNA expression and DFS. The characteristics of the patients included in the survival analysis are shown in Table 2.
Figure 2.
Flowchart demonstrating the patients excluded from the analysis.
Table 2.
Patient characteristics of the 252 patients in the TCGA cohort with available follow-up data on disease-specific survival divided by gender
| Male | Female | |
|---|---|---|
| Total cohort n (%) | 183 (100) | 69 (100) |
| Tumor stage n (%) | ||
| T2 | 62 (33.9) | 22 (31.9) |
| T3 | 95 (51.9) | 38 (55.1) |
| T4 | 26 (14.2) | 9 (13.0) |
| Tumor grade (WHO 2004) n (%) | ||
| Low grade | 17 (9.3) | 1 (1.5) |
| High grade | 164 (89.6) | 68 (98.6) |
| NA | 2 (1.1) | 0 (0) |
| Nodal status n (%) | ||
| pN+ | 61 (33.3) | 21 (30.4) |
| pN0 | 122 (66.7) | 48 (69.6) |
| Adjuvant therapy n (%) | 36 (19.7) | 13 (18.8) |
| Positive smoking history n (%) | 136 (74.3) | 39 (56.5) |
| Median age years (IQR) | 66 (41–90) | 69 (43–90) |
| Median follow-up months (IQR) | 15.6 (0.4–142.7) | 15.6 (0.6–163.2) |
| Disease-specific event n (%) | 71 (38.8) | 33 (47.8) |
| Death n (%) | 45 (24.6) | 24 (34.8) |
| AR expression n (%) | ||
| <median | 85 (46.5) | 43 (62.3) |
| ≥median | 98 (53.6) | 26 (37.7) |
| TCGA subtype n (%) | ||
| TCGA subtype I | 67 (36.6) | 22 (31.9) |
| TCGA subtype II | 47 (25.7) | 13 (18.8) |
| TCGA subtype III | 39 (21.3) | 19 (27.5) |
| TCGA subtype IV | 29 (15.9) | 13 (18.8) |
| NA | 1 (0.6) | 2 (2.9) |
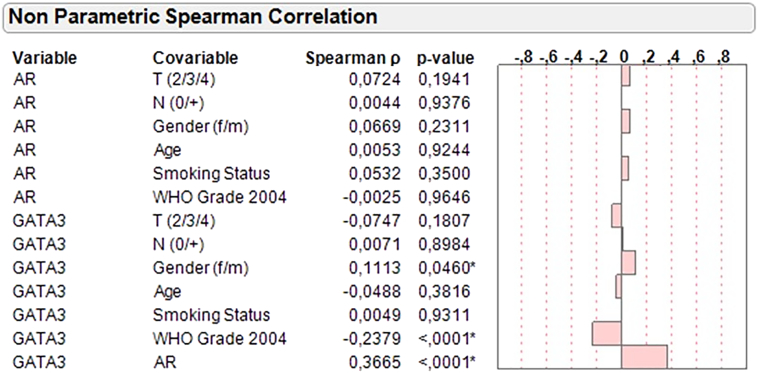
Spearman's rank correlation demonstrated a significant positive association between AR mRNA expression and GATA3 mRNA expression (rs = 0.37; P < .0001) (Supplementary Figure 2). There was no significant association between AR mRNA expression and tumor stage, grade WHO 2004, nodal status, age, gender, and smoking status in MIBC. Wilcoxon/Kruskal-Wallis testing revealed a statistically significant association between the TCGA subtype and AR mRNA expression (Chi2:46.7; P < .0001) (Figure 3). AR distribution is significantly lower in the basal subtypes III and IV when compared to the luminal subtypes (Figure 4). Interestingly, AR mRNA expression between the two basal subtypes differs almost significantly as well (P = .06).
Supplementary Figure 2.
Spearman’s rank correlation of AR and GATA3 mRNA expression.
Figure 3.
Correlation of AR mRNA expression with clinicopathological features in the TCGA cohort.
Figure 4.
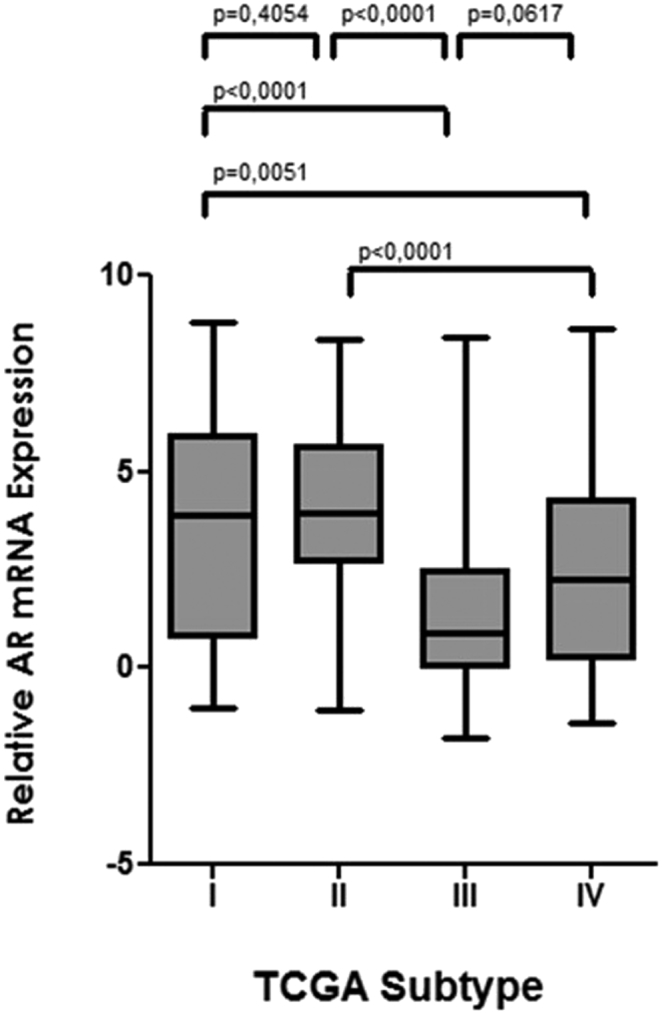
Box plot analysis of AR mRNA expression according to TCGA subtype in the TCGA cohort.
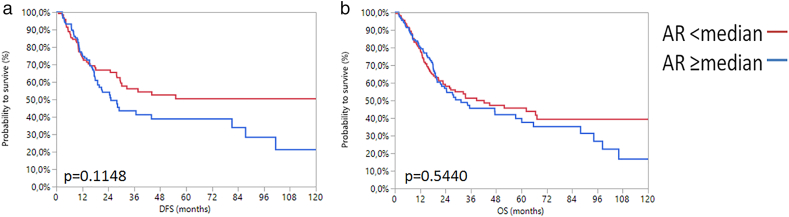
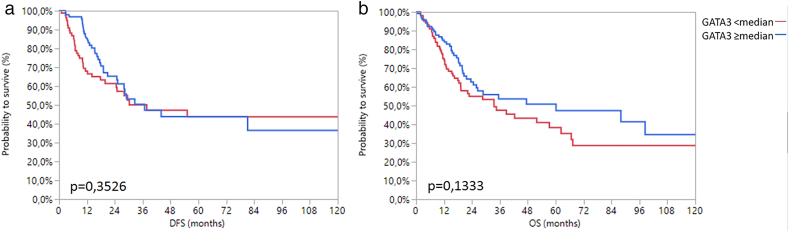
For analysis of AR expression on survival we used the median AR mRNA expression level (2.83) as the cut-off to define high and low AR expression. This way, no significant association between AR mRNA expression and DFS (P = .11) or OS (P = .54) was observed in Kaplan–Meier analysis (Supplementary Figure 3).
Supplementary Figure 3.
Kaplan-Meier analysis in the TCGA cohort for DFS (a) and OS (b) based on AR mRNA expression.
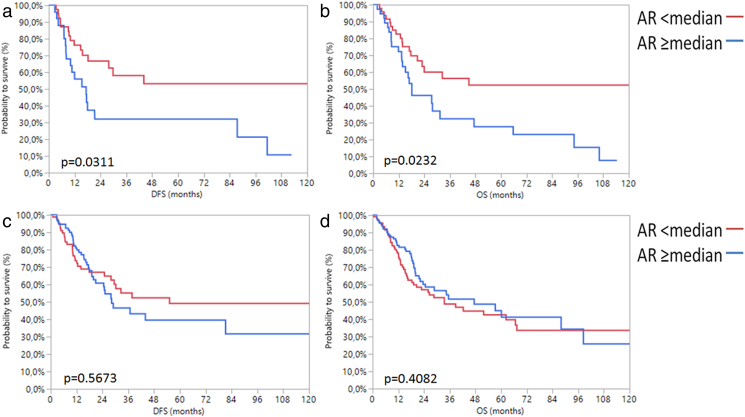
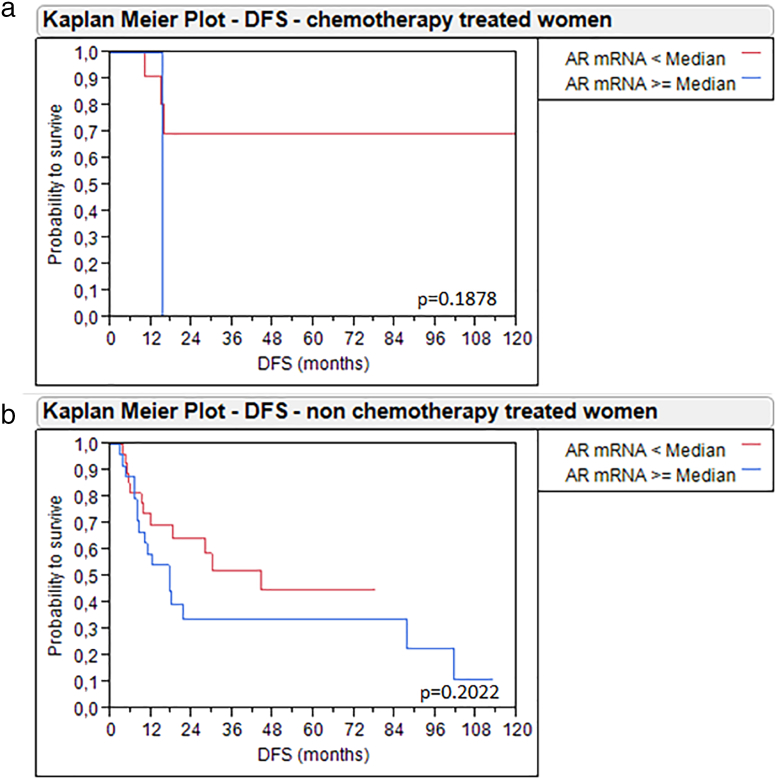
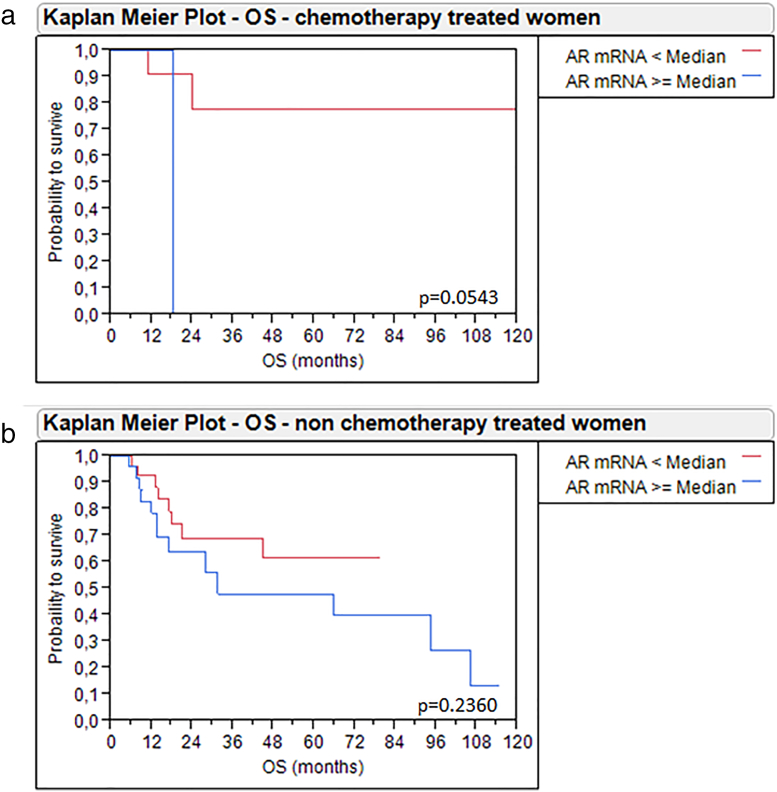
Given that gender-specific differences of the role of AR can be expected, we additionally accounted for gender in the Kaplan–Meier analysis. Women with high AR mRNA expression ≥2.83 had significantly worse DFS (P = .03) and OS (P = .02) than women with low AR mRNA expression <2.83. By contrast, men had no changes in survival depending on AR expression (Figure 5). These results may in part be attributed to adjuvant radio−/chemotherapy, as 28.6% (12/42) of the women with AR <2.83 and only 3.7% (1/27) of females with AR ≥2.83 received an adjuvant therapy, while the distribution of adjuvant therapy was more balanced in male patients (22.4% (19/85) of men with low AR and 17.9% (17/98) of men with high AR received adjuvant therapy). We tried to address this problem by separately analyzing women with and without adjuvant therapy. The subgroup of women without adjuvant chemotherapy (n = 56) only revealed a trend towards improved OS and DS when AR mRNA expression was low, which, however, was not statistically significant. The subgroup who received adjuvant therapy contained only 13 patients, making statistical analyses difficult, although a trend towards improved survival was seen here as well (Supplementary Figure 4, Supplementary Figure 5). The small size of these subgroups prevents meaningful analyses and conclusions. Therefore, a possible bias must be considered when interpreting our survival analysis of all 69 females.
Figure 5.
Kaplan–Meier analysis for DFS (a) and OS (b) in women as well as DFS (c) and OS (d) in men with MIBC depending on AR mRNA expression.
Supplementary Figure 4.
Kaplan-Meier analysis for DFS in women with MIBC treated with adjuvant chemotherapy (a) and without adjuvant chemotherapy (b) depending on AR mRNA expression.
Supplementary Figure 5.
Kaplan-Meier analysis for OS in women with MIBC treated with adjuvant chemotherapy (a) and without adjuvant chemotherapy (b) depending on AR mRNA expression.
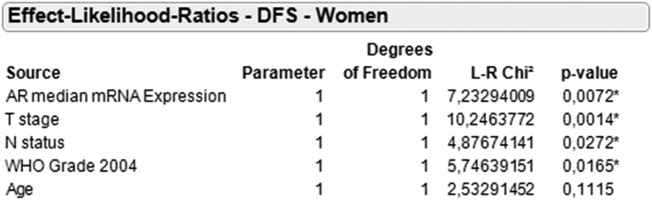
Finally, multivariable Cox's regression analysis demonstrated median AR mRNA expression in women to be an independent prognostic marker for DFS (L-R Chi2:7.23; P = .007) and OS (L-R Chi2:4.32; P = .04) when stratified for tumor stage, grade, nodal status, and age (Figure 6).
Figure 6.
Multivariable Cox's regression analysis for DFS in women stratified by median AR mRNA expression.
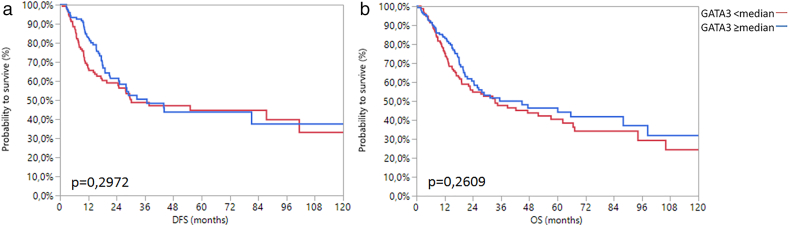
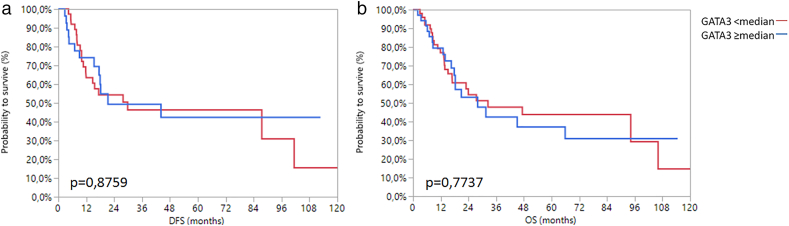
Since an association with AR mRNA expression was previously suggested, we additionally analyzed GATA3 mRNA expression in MIBC by analogy with AR. GATA3 mRNA expression was also associated with the TCGA subtype (Chi2 = 146.83; P < .0001) (Supplementary Figure 6). Unlike AR however, GATA3 mRNA expression was significantly associated with gender (Chi2 = 3.97; P = .05), tumor stage (Chi2 = 6.61; P = .03) and showed a negative association with tumor grade (Chi2 = 18.11; P < .0001). Interestingly, there was no association between GATA3 mRNA expression and survival, irrespective of whether the total or gender-specific cohorts were analyzed (Supplementary Figure 7, Supplementary Figure 8, Supplementary Figure 9).
Supplementary Figure 6.
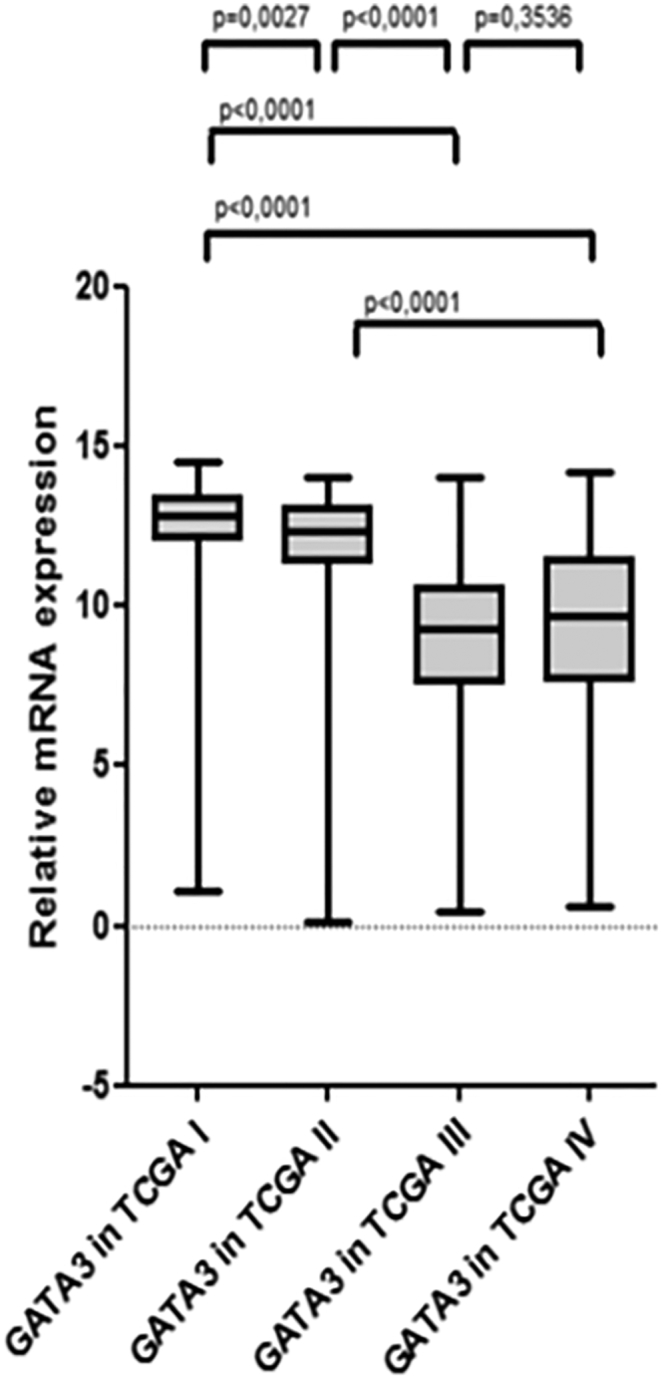
Box plot analysis of GATA3 and AR mRNA expression according to TCGA subtype.
Supplementary Figure 7.
Kaplan-Meier analysis of DFS (a) and OS (b) in the TCGA cohort with regard to median GATA3 mRNA expression.
Supplementary Figure 8.
Kaplan-Meier analysis of DFS (a) and OS (b) in men with regard to median GATA3 mRNA expression.
Supplementary Figure 9.
Kaplan-Meier analysis of DFS (a) and OS (b) in women with regard to median GATA3 mRNA expression.
Discussion
Perception of UCB has changed vastly in recent years. After over two decades of very little improvement of diagnostic and therapeutic options [28], recent studies demonstrated UCB to be a very heterogenic disease with multiple genetic mutations as well as molecular subtypes, offering new possibilities to classify and treat UCB [20], [27], [29], [30]. Moreover, because of gender-specific differences of UCB, sexual hormones in general and AR in particular have been discussed as a possible factor in the genesis and progression of UCB [6]. However, because of conflicting results, the role of AR in UCB remains unclear [8], [13], [14], [15]. The differing results might partially be attributed to immunohistochemistry as the main means of determining AR expression in most studies, as immunohistochemistry is associated with high inter-observer variability [16]. To avoid the technical limitations of immunohistochemistry in the assessment of AR protein expression in UCB, we recently measured AR mRNA expression in stage T1 UCB using reverse transcription quantitative real-time polymerase chain reaction (RT-qPCR) [17]. We found that AR mRNA expression is associated with better survival in stage T1 UCB.
Even though immunohistochemistry based studies had contradictory results, they consistently report a changing role of AR in UCB with increasing stage. Therefore, in the present study, we retrospectively investigated AR mRNA expression in all stages of UCB within two independent cohorts. In the Aarhus cohort, AR mRNA expression was higher in lower stages, which is most prominent between non-muscle-invasive and muscle-invasive disease. There were no significant differences in AR mRNA expression when comparing stages T2 – T4, which was also the case in the TCGA cohort. Unfortunately, given that no data on progression was available in both cohorts, it cannot be concluded that AR mRNA expression changes during progression from NMIBC to MIBC. However, these results suggest a different role of AR in NMIBC and MIBC, while there seem to be no relevant additional changes of AR expression within the different stages of MIBC. The current results are comparable to the findings of Boorjan et al. [14].
The high expression of AR mRNA in NMIBC might also be of therapeutic relevance. Several preclinical studies demonstrated an improved BCG efficacy in the presence of antiandrogens [31], [32]. AR degradation was shown to recruit monocytes and macrophages that promote BCG attachment to UCB cells which eventually enhance BCG-induced UCB cell death through TNF-α release [31]. Unfortunately, lacking any information on BCG therapy and survival, we are not able to draw any conclusions from our current results. However, high AR expression in NMIBC might be responsible for patients not responding to BCG therapy. Further clinical studies will be necessary to address this topic.
Since the available clinicopathological data in the Aarhus cohort were limited, the analysis was focused on the TCGA cohort, which comprised only patients with stage T2 – T4 MIBC. The results were mostly negative. There was no significant association between AR mRNA expression with tumor stage, grade or nodal status. Interestingly, there was also no association between AR expression and age, as change in AR expression with increasing age might be assumed [33], which is not the case in the current study. Moreover, no association was found between AR expression and smoking status, which was suggested by a previous study [16].
The only statistically significant association was observed between AR mRNA expression and GATA3 mRNA expression as well as the TCGA subtype.
The relation between AR and GATA3 was examined because an association was implicated by previous studies [19], [22]. Previous investigations demonstrated a protective role of GATA3 expression in the urinary bladder, as GATA3 was shown to be down-regulated in urothelial carcinoma, high grade urothelial carcinoma and MIBC when compared to normal urothelium, low grade urothelial carcinoma and NMIBC, respectively [22]. The current results are in concordance with previous findings, as we also found an inverse association between GATA3 expression and tumor grade. Moreover, Li et al. showed that androgens are capable of decreasing GATA3 expression in normal urothelial cell lines via AR mediation, while no effect of androgens on GATA3 expression in neoplastic urothelial cells was observed [19]. This also coincides with our results, since AR and GATA3 expression were positively correlated in MIBC in the current study, meaning that AR had no negative effect on GATA3 expression in bladder cancer. GATA3 might have a different role in UCB when compared to normal urothelium. Interestingly, although GATA3 demonstrated an association with several clinicopathological features, it had no prognostic relevance regarding survival. However, in concordance with previous studies [20], [29], we observed a significantly higher expression in the luminal TCGA subtypes I and II, confirming GATA3 as an important marker to distinguish luminal from basal subtypes.
Analysis of the association of AR mRNA expression with the TCGA subtypes in MIBC revealed a significantly lower AR mRNA expression in the two basal subtypes III and IV when compared to the luminal subtypes I and II. The lowest AR expression was in TCGA subtype III, which is mainly characterized by high expression of keratins, like KRT5, KRT6a and KRT14 [27]. The current results in MIBC are in contrast to our previous findings in stage T1 bladder cancer, which showed a statistically significant positive association between high mRNA expression of AR and KRT5, both of which were associated with better survival [17]. On the other hand, other molecular analyses found the basal subtype to be more aggressive than the luminal subtype in MIBC [20]. Taken together, these findings implicate a lower AR expression in MIBC, which might contribute to the worse outcome of basal subtypes in MIBC, while AR targeted therapies for luminal MIBC might be considered a possible therapeutic option in the future.
Overall, the most interesting finding of the current analysis is the gender-specific influence of AR mRNA expression on survival. When using AR expression at median level as a cut-off to define high and low AR mRNA expression, 53.6% of male patients and 37.7% of female patients with MIBC had high expression. While AR expression had no effect on survival in men, females expressing AR mRNA above median level demonstrated significantly worse DFS and OS. These results may in part be attributed to adjuvant radio−/chemotherapy, as 27.9% of females with low AR expression and only 3.9% of females with high AR expression received an adjuvant therapy, which has to be considered when drawing conclusions from the current results. We tried to address this problem by separately analyzing women with and without adjuvant therapy. This way, a trend towards improved survival was seen in both subgroups when AR mRNA expression was below median level, although the results were not statistically significant. However, the small size of these subgroups prevents meaningful analyses and conclusions. Therefore, a possible bias must be considered when interpreting our survival analysis of all 69 females. Nevertheless, considering the poor response of UCB to chemotherapy [34], [35], the possible gender-specific role of AR in female patients with MIBC should not be disregarded and should be validated in further studies. The gender-specific difference regarding the association of AR mRNA expression and survival would be especially surprising, because AR mRNA expression does not differ significantly between men and women. This effect might be due to distinct hormone levels between men and women as well as cross-reactions between AR and other hormonal receptors, which has been suggested in breast cancer [36]. This way, a different role of high AR expression in women and men is thinkable, even though AR mRNA expression does not differ significantly between genders. Nevertheless, the reasons for the different results in females and males remain unclear and need further investigation.
Finally, as in NMIBC, the therapeutic relevance of AR expression in MIBC still has to be determined. Recent studies demonstrated a better outcome for patients with UCB when treated with antiandrogens for concomitant prostate cancer [37]. Another study suggested an improved efficacy of platinum-based chemotherapy when given together with antiandrogens [38]. Since AR expression does not decrease with higher tumor stages according to our results, patients with stage T3 or T4 MIBC might still benefit from an additional antiandrogen therapy accompanying chemotherapy. Further studies are necessary to clarify this topic.
There are several limitations to this study. First, there are the limitations associated with a retrospective study. Some important information, like most clinical features in the Aarhus cohort or the treatment modalities in both cohorts were missing. We decided to overcome this problem by including only patients with a documented pN status as a surrogate for a surgical therapeutic approach in the TCGA cohort, however the exact type and outcome of the surgery remains unclear, which has to be accounted for when interpreting our results regarding survival. Another limitation is the small size and uneven composition of the Aarhus cohort while the disproportionate distribution of adjuvant therapy in females in the TCGA cohort might lead to a bias in the survival analysis. Moreover, the diversity of the two cohorts regarding population, available data and methods used for AR mRNA quantification means that a direct comparison of the two cohorts is not possible. Another possible limitation of mRNA quantification is the potential contamination with non-neoplastic urothelium. While we used only samples containing more than 50% tumor cells by analogy with previous studies in breast cancer [24], [25], [26], a contamination cannot be entirely excluded. Finally, no data was available on immunohistochemical staining of the analyzed genes, which, however, remains the most readily available form of detection of molecular markers.
In conclusion, by analyzing AR mRNA expression in UCB within two different cohorts a significantly lower AR mRNA expression was demonstrated in MIBC when compared to NMIBC, with no further differences in AR mRNA expression within the various stages of MIBC. Moreover, AR mRNA expression demonstrated a gender-specific impact on outcome. Females expressing AR mRNA above median level had significantly worse DFS. Further studies with larger cohorts are necessary to clarify the gender-dependent role of AR in UCB.
The following are the supplementary data related to this article.
Acknowledgments
Acknowledgements
D.S. is supported by a Ferdinand Eisenberger grant of the German Society of Urology (Deutsche Gesellschaft für Urologie), grant ID SiD1/FE-16.
Conflict of Interest
The authors declare to have no competing interests.
Contributor Information
Danijel Sikic, Email: danijel.sikic@uk-erlangen.de.
Ralph M. Wirtz, Email: ralph.wirtz@STRATIFYER.de.
Sven Wach, Email: sven.wach@uk-erlangen.de.
Lars Dyrskjøt, Email: lars@clin.au.dk.
Philipp Erben, Email: philipp.erben@medma.uni-heidelberg.de.
Christian Bolenz, Email: christian.bolenz@uniklinik-ulm.de.
Johannes Breyer, Email: johannes.breyer@ukr.de.
Wolfgang Otto, Email: wolfgang.otto@ukr.de.
Katherine A. Hoadley, Email: hoadley@med.unc.edu.
Seth P. Lerner, Email: slerner@bcm.edu.
Markus Eckstein, Email: markus.eckstein@uk-erlangen.de.
Arndt Hartmann, Email: arndt.hartmann@uk-erlangen.de.
Bastian Keck, Email: bastian.keck@web.de.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Chavan S, Bray F, Lortet-Tieulent J, Goodman M, Jemal A. International variations in bladder cancer incidence and mortality. Eur Urol. 2014;66(1):59–73. doi: 10.1016/j.eururo.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Hartge P, Harvey EB, Linehan WM, Silverman DT, Sullivan JW, Hoover RN, Fraumeni JF., Jr. Unexplained excess risk of bladder cancer in men. J Natl Cancer Inst. 1990;82(20):1636–1640. doi: 10.1093/jnci/82.20.1636. [DOI] [PubMed] [Google Scholar]
- 4.Scosyrev E, Noyes K, Feng C, Messing E. Sex and racial differences in bladder cancer presentation and mortality in the US. Cancer. 2009;115(1):68–74. doi: 10.1002/cncr.23986. [DOI] [PubMed] [Google Scholar]
- 5.Burge F, Kockelbergh R. Closing the Gender Gap: Can We Improve Bladder Cancer Survival in Women? - A Systematic Review of Diagnosis, Treatment and Outcomes. Urol Int. 2016 doi: 10.1159/000449256. [DOI] [PubMed] [Google Scholar]
- 6.Dobruch J, Daneshmand S, Fisch M, Lotan Y, Noon AP, Resnick MJ, Shariat SF, Zlotta AR, Boorjian SA. Gender and Bladder Cancer: A Collaborative Review of Etiology, Biology, and Outcomes. Eur Urol. 2016;69(2):300–310. doi: 10.1016/j.eururo.2015.08.037. [DOI] [PubMed] [Google Scholar]
- 7.Lombard AP, Mudryj M. The emerging role of the androgen receptor in bladder cancer. Endocr Relat Cancer. 2015;22(5):R265–R277. doi: 10.1530/ERC-15-0209. [DOI] [PubMed] [Google Scholar]
- 8.Zheng Y, Izumi K, Yao JL, Miyamoto H. Dihydrotestosterone upregulates the expression of epidermal growth factor receptor and ERBB2 in androgen receptor-positive bladder cancer cells. Endocr Relat Cancer. 2011;18(4):451–464. doi: 10.1530/ERC-11-0010. [DOI] [PubMed] [Google Scholar]
- 9.Miyamoto H, Yang Z, Chen YT, Ishiguro H, Uemura H, Kubota Y, Nagashima Y, Chang YJ, Hu YC, Tsai MY. Promotion of bladder cancer development and progression by androgen receptor signals. J Natl Cancer Inst. 2007;99(7):558–568. doi: 10.1093/jnci/djk113. [DOI] [PubMed] [Google Scholar]
- 10.Imada S, Akaza H, Ami Y, Koiso K, Ideyama Y, Takenaka T. Promoting effects and mechanisms of action of androgen in bladder carcinogenesis in male rats. Eur Urol. 1997;31(3):360–364. doi: 10.1159/000474484. [DOI] [PubMed] [Google Scholar]
- 11.Hsu JW, Hsu I, Xu D, Miyamoto H, Liang L, Wu XR, Shyr CR, Chang C. Decreased tumorigenesis and mortality from bladder cancer in mice lacking urothelial androgen receptor. Am J Pathol. 2013;182(5):1811–1820. doi: 10.1016/j.ajpath.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin C, Yin Y, Stemler K, Humphrey P, Kibel AS, Mysorekar IU, Ma L. Constitutive beta-catenin activation induces male-specific tumorigenesis in the bladder urothelium. Cancer Res. 2013;73(19):5914–5925. doi: 10.1158/0008-5472.CAN-12-4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mashhadi R, Pourmand G, Kosari F, Mehrsai A, Salem S, Pourmand MR, Alatab S, Khonsari M, Heydari F, Beladi L. Role of steroid hormone receptors in formation and progression of bladder carcinoma: a case-control study. Urol J. 2014;11(6):1968–1973. [PubMed] [Google Scholar]
- 14.Boorjian S, Ugras S, Mongan NP, Gudas LJ, You X, Tickoo SK, Scherr DS. Androgen receptor expression is inversely correlated with pathologic tumor stage in bladder cancer. Urology. 2004;64(2):383–388. doi: 10.1016/j.urology.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 15.Tuygun C, Kankaya D, Imamoglu A, Sertcelik A, Zengin K, Oktay M, Sertcelik N. Sex-specific hormone receptors in urothelial carcinomas of the human urinary bladder: a comparative analysis of clinicopathological features and survival outcomes according to receptor expression. Urol Oncol. 2011;29(1):43–51. doi: 10.1016/j.urolonc.2009.01.033. [DOI] [PubMed] [Google Scholar]
- 16.Mir C, Shariat SF, van der Kwast TH, Ashfaq R, Lotan Y, Evans A, Skeldon S, Hanna S, Vajpeyi R, Kuk C. Loss of androgen receptor expression is not associated with pathological stage, grade, gender or outcome in bladder cancer: a large multi-institutional study. BJU Int. 2011;108(1):24–30. doi: 10.1111/j.1464-410X.2010.09834.x. [DOI] [PubMed] [Google Scholar]
- 17.Sikic D, Breyer J, Hartmann A, Burger M, Erben P, Denzinger S, Eckstein M, Stohr R, Wach S, Wullich B. High Androgen Receptor mRNA Expression Is Independently Associated with Prolonged Cancer-Specific and Recurrence-Free Survival in Stage T1 Bladder Cancer. Transl Oncol. 2017;10(3):340–345. doi: 10.1016/j.tranon.2017.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim S, Moon BI, Lim W, Park S, Cho MS, Sung SH. Expression patterns of GATA3 and the androgen receptor are strongly correlated in patients with triple-negative breast cancer. Hum Pathol. 2016;55:190–195. doi: 10.1016/j.humpath.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 19.Li Y, Ishiguro H, Kawahara T, Miyamoto Y, Izumi K, Miyamoto H. GATA3 in the urinary bladder: suppression of neoplastic transformation and down-regulation by androgens. Am J Cancer Res. 2014;4(5):461–473. [PMC free article] [PubMed] [Google Scholar]
- 20.Choi W, Porten S, Kim S, Willis D, Plimack ER, Hoffman-Censits J, Roth B, Cheng T, Tran M, Lee I-L. Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer Cell. 2014;25(2):152–165. doi: 10.1016/j.ccr.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lerner SP, McConkey DJ, Hoadley KA, Chan KS, Kim WY, Radvanyi F, Hoglund M, Real FX. Bladder Cancer Molecular Taxonomy: Summary from a Consensus Meeting. Bladder Cancer. 2016;2(1):37–47. doi: 10.3233/BLC-150037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miyamoto H, Izumi K, Yao JL, Li Y, Yang Q, McMahon LA, Gonzalez-Roibon N, Hicks DG, Tacha D, Netto GJ. GATA binding protein 3 is down-regulated in bladder cancer yet strong expression is an independent predictor of poor prognosis in invasive tumor. Hum Pathol. 2012;43(11):2033–2040. doi: 10.1016/j.humpath.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 23.Dyrskjot L, Zieger K, Kruhoffer M, Thykjaer T, Jensen JL, Primdahl H, Aziz N, Marcussen N, Moller K, Orntoft TF. A molecular signature in superficial bladder carcinoma predicts clinical outcome. Clin Cancer Res. 2005;11(11):4029–4036. doi: 10.1158/1078-0432.CCR-04-2095. [DOI] [PubMed] [Google Scholar]
- 24.Stefanovic S, Wirtz R, Deutsch TM, Hartkopf A, Sinn P, Varga Z, Sobottka B, Sotiris L, Taran FA, Domschke C. Tumor biomarker conversion between primary and metastatic breast cancer: mRNA assessment and its concordance with immunohistochemistry. Oncotarget. 2017;8(31):51416–51428. doi: 10.18632/oncotarget.18006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barry WT, Kernagis DN, Dressman HK, Griffis RJ, Hunter JD, Olson JA, Marks JR, Ginsburg GS, Marcom PK, Nevins JR. Intratumor heterogeneity and precision of microarray-based predictors of breast cancer biology and clinical outcome. J Clin Oncol. 2010;28(13):2198–2206. doi: 10.1200/JCO.2009.26.7245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laible M, Schlombs K, Kaiser K, Veltrup E, Herlein S, Lakis S, Stohr R, Eidt S, Hartmann A, Wirtz RM. Technical validation of an RT-qPCR in vitro diagnostic test system for the determination of breast cancer molecular subtypes by quantification of ERBB2, ESR1, PGR and MKI67 mRNA levels from formalin-fixed paraffin-embedded breast tumor specimens. BMC Cancer. 2016;16:398. doi: 10.1186/s12885-016-2476-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Network CGAR Comprehensive molecular characterization of urothelial bladder carcinoma. Nature. 2014;507(7492):315–322. doi: 10.1038/nature12965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, Dawson N, O'Donnell PH, Balmanoukian A, Loriot Y. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387(10031):1909–1920. doi: 10.1016/S0140-6736(16)00561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Damrauer JS, Hoadley KA, Chism DD, Fan C, Tiganelli CJ, Wobker SE, Yeh JJ, Milowsky MI, Iyer G, Parker JS. Intrinsic subtypes of high-grade bladder cancer reflect the hallmarks of breast cancer biology. Proc Natl Acad Sci U S A. 2014;111(8):3110–3115. doi: 10.1073/pnas.1318376111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen C, Qi XJ, Cao YW, Wang YH, Yang XC, Shao SX, Niu HT. Bladder Tumor Heterogeneity: The Impact on Clinical Treatment. Urol Int. 2015;95(1):1–8. doi: 10.1159/000370165. [DOI] [PubMed] [Google Scholar]
- 31.Shang Z, Li Y, Zhang M, Tian J, Han R, Shyr CR, Messing E, Yeh S, Niu Y, Chang C. Antiandrogen Therapy with Hydroxyflutamide or Androgen Receptor Degradation Enhancer ASC-J9 Enhances BCG Efficacy to Better Suppress Bladder Cancer Progression. Mol Cancer Ther. 2015;14(11):2586–2594. doi: 10.1158/1535-7163.MCT-14-1055-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen F, Langenstroer P, Zhang G, Iwamoto Y, See W. Androgen dependent regulation of bacillus Calmette-Guerin induced interleukin-6 expression in human transitional carcinoma cell lines. J Urol. 2003;170(5):2009–2013. doi: 10.1097/01.ju.0000092238.15685.10. [DOI] [PubMed] [Google Scholar]
- 33.Prins GS, Jung MH, Vellanoweth RL, Chatterjee B, Roy AK. Age-dependent expression of the androgen receptor gene in the prostate and its implication in glandular differentiation and hyperplasia. Dev Genet. 1996;18(2):99–106. doi: 10.1002/(SICI)1520-6408(1996)18:2<99::AID-DVG2>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 34.Yafi FA, North S, Kassouf W. First- and second-line therapy for metastatic urothelial carcinoma of the bladder. Curr Oncol. 2011;18(1):e25–e34. doi: 10.3747/co.v18i1.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Witjes JA, Compérat E, Cowan NC, De Santis M, Gakis G, Lebret T, Ribal MJ, Van der Heijden AG, Sherif A, Urology EAo EAU guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2013 guidelines. Eur Urol. 2014;65(4):778–792. doi: 10.1016/j.eururo.2013.11.046. [DOI] [PubMed] [Google Scholar]
- 36.Schweizer MT, Yu EY. AR-Signaling in Human Malignancies: Prostate Cancer and Beyond. Cancer. 2017;9(1) doi: 10.3390/cancers9010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Izumi K, Taguri M, Miyamoto H, Hara Y, Kishida T, Chiba K, Murai T, Hirai K, Suzuki K, Fujinami K. Androgen deprivation therapy prevents bladder cancer recurrence. Oncotarget. 2014;5(24):12665–12674. doi: 10.18632/oncotarget.2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kashiwagi E, Ide H, Inoue S, Kawahara T, Zheng Y, Reis LO, Baras AS, Miyamoto H. Androgen receptor activity modulates responses to cisplatin treatment in bladder cancer. Oncotarget. 2016 doi: 10.18632/oncotarget.9994. [DOI] [PMC free article] [PubMed] [Google Scholar]