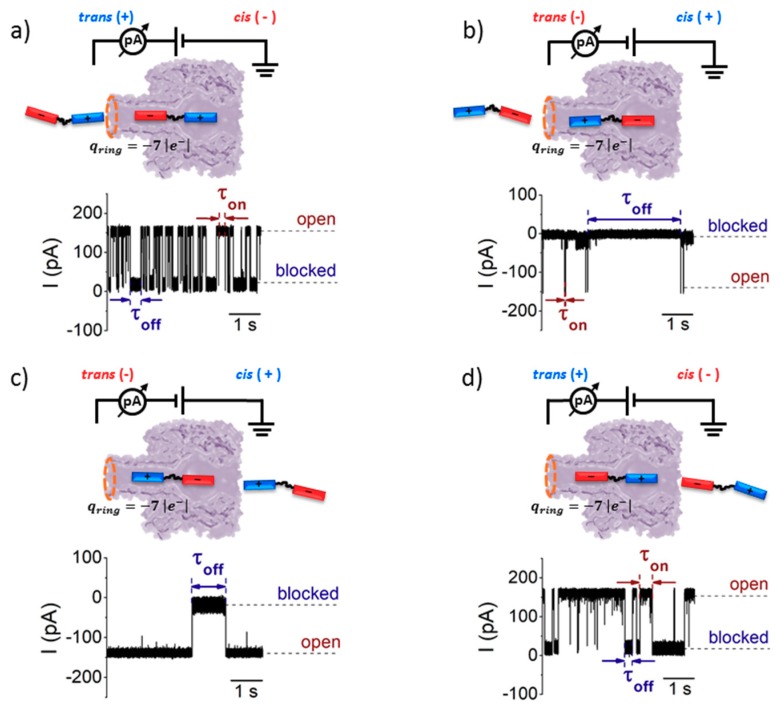
Figure 5.
Sketch of the protocol aimed at studying the sidedness dependence of the S6-containing peptides-α-HL interactions. The peptide was added to the trans side of the membrane, and positive (a) or negative ΔVs (b) were employed to drive the peptide vectorially, with either the R12 or E12 moiety toward the nanopore’s β-barrel. The selected recordings below illustrate the details of current fluctuation through an α-HL inserted into a planar lipid bilayer, induced by the reversible peptide binding. The times between two consecutive peptide binding events (τon) and of the peptide transient residence inside the nanopore (τoff) are also shown. (c,d) Precise similar chains of events are represented, except that the peptide was present on the cis side of the membrane. Note that, in this case, to drive the peptide inside the nanopore’s vestibule with the same orientation as above (i.e., R12 or E12 moiety head on), opposite polarities of the applied ΔVs, as compared to those used in (a,b), are needed.