-
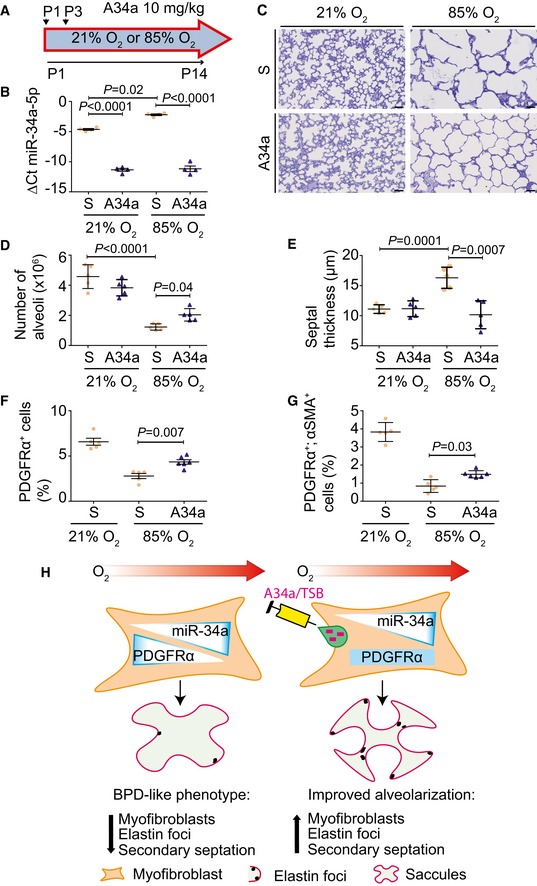
A
Schematic illustration of the antimiR administration protocol.
-
B
Quantitative RT–PCR detection of miR‐34a‐5p levels in wild‐type mouse pups at post‐natal day (P)14 that had been treated either with a scrambled antimiR (S), or antimiR‐34a (A34a), during normal (21% O2) and aberrant (85% O2) alveolarization (n = 4 animals for each group).
-
C
Qualitative analysis of lung structure in Richardson‐stained plastic‐embedded lung sections from wild‐type mouse pups at post‐natal day (P)14, treated with either scrambled antimiR (S), or antimiR‐34a (A34a), during normal and aberrant alveolarization (scale bar, 50 μm). Data are representative of three or more experiments.
-
D
Quantification of total number of alveoli by design‐based stereology in wild‐type mouse pups at post‐natal day (P)14, treated with either scrambled antimiR (S), or antimiR‐34a (A34a), during normal and aberrant lung alveolarization (n = 5 animals for each group).
-
E
Quantification of mean septal thickness by design‐based stereology in wild‐type mouse pups at post‐natal day (P)14, treated with either scrambled antimiR (S), or antimiR‐34a (A34a), during normal and aberrant alveolarization (n = 5 animals for each group).
-
F
Quantitative analysis of PDGFRα+ cells by flow cytometry, in lungs from wild‐type mouse pups at P5, treated with either scrambled antimiR (S), or antimiR‐34a (A34a), during normal and aberrant alveolarization (n = 5 animals for each group).
-
G
Quantitative analysis of PDGFRα+/αSMA+ cells by flow cytometry, in lungs from wild‐type mouse pups at P5, treated with either scrambled antimiR (S), or antimiR‐34a (A34a), during normal and aberrant alveolarization (n = 5 animals for each group).
-
H
Schematic illustration of the role and translational scope of the miR‐34a/Pdgfra interaction during arrested lung alveolarization. Hyperoxia drives miR‐34a expression in myofibroblasts, downregulating PDGFRα expression and reducing PDGFRα+ cell abundance, causing the perturbed elastin fiber production and blunted alveolarization seen in bronchopulmonary dysplasia (BPD). The effects of hyperoxia are attenuated when miR‐34a function is blocked with an antimiR (A34a) or when the miR‐34a/Pdgfra interaction is disturbed with a target site blocker (TSB).
Data information: Data represent mean ± SD.
modification.