Figure 6.
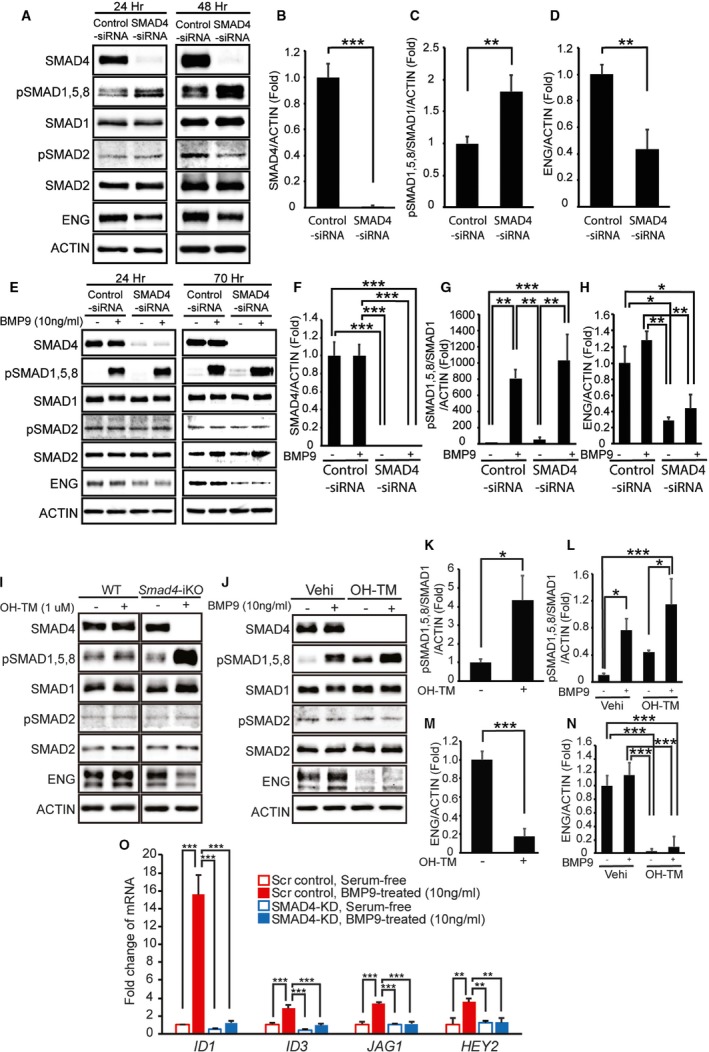
SMAD4 depletion causes loss of canonical ALK1/endoglin (ENG) signaling. A through D, Western blot analyses of SMAD4, phosphorylated and total R‐SMADs, and ENG in human umbilical vein endothelial cells (HUVECs) treated with scrambled control small interfering RNA (siRNA) or SMAD4‐siRNA for 24 and 48 hours in normal culture media (A). Quantification of SMAD4 proteins (B), pSMAD1, 5, and 8 levels (C), and ENG proteins (D) at 48 hours after siRNA transfection. E through H, Protein levels of SMAD4, phosphorylated and total R‐SMADs, and ENG in control and SMAD4‐KD HUVECs treated with or without bone morphogenetic protein 9 (BMP9) in serum‐starved condition at 24 and 70 hours after siRNA transfection (E). Quantification shows a >95% decrease of SMAD4 (F), responses to BMP9 (G), and reduction of ENG proteins (H) in SMAD4‐KD HUVECs 70 hours after transfection. 10 ng/mL of human BMP9 was treated for 2 hours after serum starvation for 16 hours. I through N, Protein levels of SMAD4, phosphorylated and total R‐SMADs, and ENG in primary pulmonary endothelial cells (pECs) isolated from wild‐type (WT) and Smad4‐inducible knockout (iKO) mice (I). Treatment with 4 hydroxyl‐tamoxifen (OH‐TM) did not affect SMAD4 and R‐SMAD expressions in WT cells but abolished SMAD4 expression and elevated pSMAD1, 5, and 8 levels in Smad4‐iKO cells (I and K). In serum‐free condition, SMAD1, 5, and 8 are activated by BMP9 treatment in both SMAD4‐depleted and normal pECs (J and L). ENG protein level is decreased in Smad4‐iKO pECs (I, J, M, and N). O, Quantitative reverse transcription polymerase chain reaction demonstrates that responses to BMP9 in upregulating BMP9‐ALK1 downstream genes ID1,ID3,JAG1, and HEY2 are markedly attenuated in SMAD4‐depleted HUVECs. ACTIN was used for normalization. All data represent mean±SD. *P<0.05, **P<0.01, ***P<0.001. Scr indicates scrambled‐siRNA; Vehi, vehicle.
