Table 3.
Substrate scope of alkyne derivativesa.
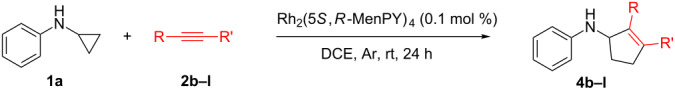 | |||||
| Entry | Substrate | Product | Yieldb(%) | ||
| 1 |  |
2b | N.D. | 4b | N.R. |
| 2 |  |
2c |  |
4c | <10 |
| 3 | 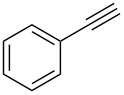 |
2d | 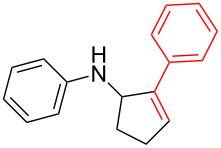 |
4d | 65 |
| 4 | 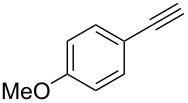 |
2e | 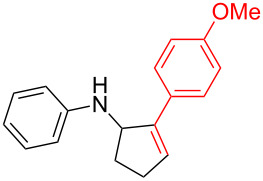 |
4e | 64 |
| 5 | 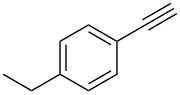 |
2f | 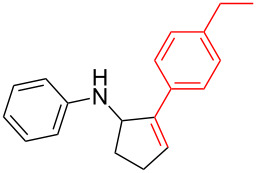 |
4f | 65 |
| 6 | 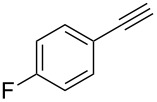 |
2g | 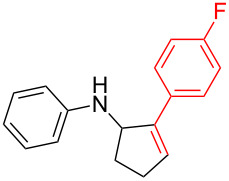 |
4g | 64 |
| 7 |  |
2h | 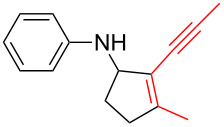 |
4h | 31 |
| 8c | 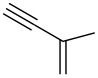 |
2i | 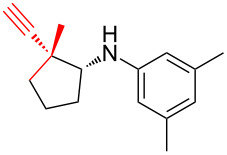 |
4id,e | 45 (17:83)f |
| 9 | 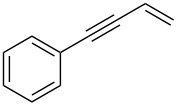 |
2j |
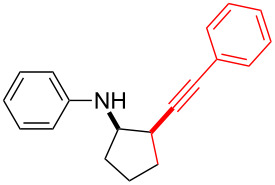 , ,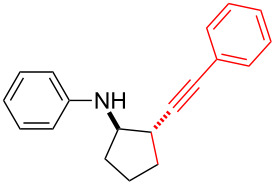
|
4j | 41 (30:70)f |
| 10 | 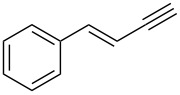 |
2k | 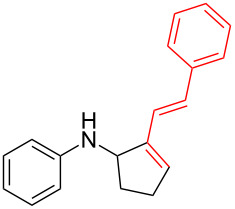 |
4k | 44 |
| 11c | 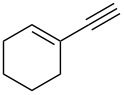 |
2l | 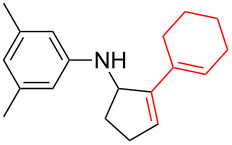 |
4ld | 40 |
aReaction conditions: 1a (1 mmol, 0.2 M in degassed solvent), 2b–l (5 mmol), catalyst (0.1 mol %) at room temperature for 24 h unless otherwise noted. bIsolated yield. cN-Arylaminocyclopropane 1d was used instead of 1a. dMajor isomer shown. eIsomer ratio 89:11. fDiastereoisomeric ratios (cis/trans) were determined by 1H NMR spectroscopy of the crude products.
