Figure 1.
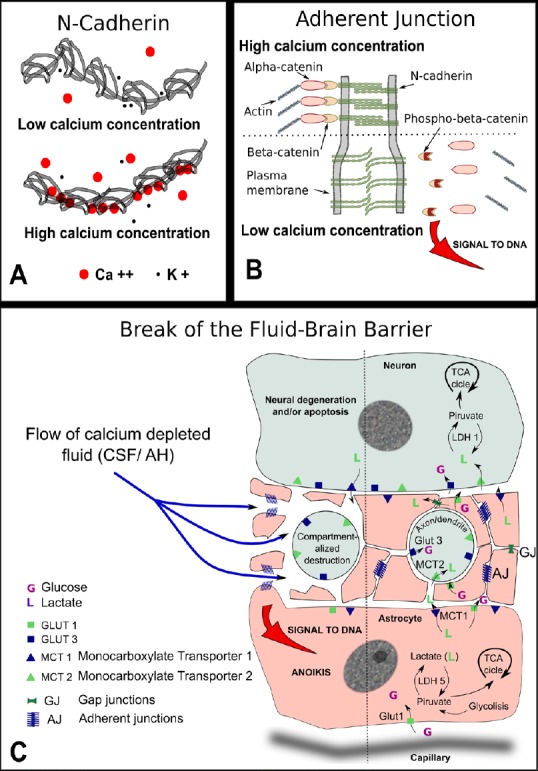
Pathogeny of the break of the fluid-brain barrier (FBB).
(A) Schematic representation of N-cadherin molecule: five identical subunits form a chain in the extracellular portion of the molecule that is the one shown here. In the presence of low calcium ion concentration, the potassium ion competes with calcium to occupy intramolecular positions. As the potassium ion is small compared to calcium the chain remains loose with ample movements among the five components. In the presence of high calcium concentration, the calcium ion occupies the molecular sockets and confers rigidity to the chain. Only rigid chains group in pairs and are able to connect with the cadherin chains of the opposing membrane. (B) Drawing of an adherent junction: With high calcium concentration, the opposing extracellular domains of N-cadherin engage in homotypic binding. Calcium ion neutralizes the negative charges of branched macromolecules of sialoglycoproteins also present at the junction (not shown). The intracellular domain of N-cadherin is attached to actin filaments with the help of two connected molecules of alpha and β-catenins. When the concentration of calcium is reduced in the extracellular cleft, the negative charges of sialic acid repel both membranes increasing the gap, β-catenin is phosphorylated to phospho-β-catenin and freed from attachment to the actin net. Phospho-β-catenin accumulates at and near the cell nucleus. If the signal is strong enough the anoikis form of apoptosis is triggered. (C) Drawing depicting possible consequences of a break of the FBB: the flow of calcium-depleted fluid in the neuropil, the prelaminar region of the optic nerve, or the gray matter in the spinal cord, will share some common features. Neuronal extensions, either axons or dendrites, are surrounded by myriads of fine astrocytic prolongations kept in place by numerous adherent junctions. A drop in calcium concentration will loosen the homotypic binding of N-cadherin with two significant consequences. Firstly, the widening of the intercellular cleft will interfere with the shuttle of lactate and other important metabolic molecules. Secondly, if the signal of the adherent junctions detachment is strong enough, phospho-β-catenin will accumulate at and near the cell nucleus and will trigger a type of apoptosis that takes place frequently during development and is known as anoikis. Anoikis, that is meant to signify ‘without residence’, forces the complete detachment from the tissue and leads to the cell auto destruction without the activation of inflammation. Both processes would impinge on the neuronal prolongations, axons and/or dendrites depending on the tissue involved, triggering a process of compartmentalized auto destruction. Consequently, the soma of the neuron would undergo chronic neurodegenerative changes that might eventually lead to apoptosis. TCA: Tricarboxylic acid cycle; CSF: cerebrospinal fluid; AH: aqueous humor; GLUT: glucose transporter.
