Abstract
Subsequent to a peripheral nerve injury, there are changes in gene expression within the dorsal root ganglia in response to the damage. This review selects factors which are well-known to be vital for inflammation, cell death and nociception, and highlights how alterations in their gene expression within the dorsal root ganglia can affect functional recovery. The majority of studies used polymerase chain reaction within animal models to analyse the dynamic changes following peripheral nerve injuries. This review aims to highlight the factors at the gene expression level that impede functional recovery and are hence are potential targets for therapeutic approaches. Where possible the experimental model, specific time-points and cellular location of expression levels are reported.
Keywords: Gene expression, polymerase chain reaction, dorsal root ganglia, inflammation, nociception, cell death, peripheral nerve injury, Schwann cells, satellite glial cells, nerve regeneration
Introduction
Overview of peripheral nerve injury
Unlike the central nervous system, the peripheral nervous system has the intrinsic ability for regeneration of neurons following damage (Huebner and Strittmatter, 2009). This is important as the peripheral nervous system lacks the same degree of protection of the central nervous system, and transmits vital information which allows for movement, touch perception and the autonomic control of the activity of organs. However, various factors can cause an imperfect recovery, such as high levels of inflammation and cell death, along with persistent pain (Scheib and Höke, 2013). Therefore, surgical intervention is sometimes required to help improve neuronal regeneration and regain functionality.
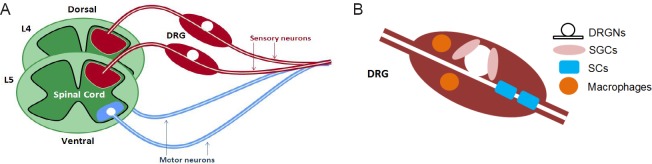
The mammalian peripheral nervous system consists of a vast network of sensory and motor neurons which communicate with the central nervous system. The sensory neurons project from the periphery to the dorsal horn of the spinal cord and provide information about the environment. In general, the sensory neurons consist of a bifurcated axon and consist of a peripheral and a central axon extension (Figure 1). The cell bodies of the sensory neurons are located in the dorsal root ganglia (DRG) which are located distal to the spinal cord. In contrast, the motor neurons project from the spinal ventral horn and receive inputs from the sensory neurons or the central nervous system.
Figure 1.
Schematic diagram of dorsal root ganglion (DRG) structure and cell types composition.
(A) A diagram of a DRG. The sensory neurons cell body is located within the DRG, with central and peripheral axon extensions. The motor neurons project out from the ventral horn of the spinal cord and combine with the peripheral sensory neurons to form the sciatic nerve. (B) An illustration of the prominent cells within the DRG. The satellite glial cells (SGCs) surround the neuronal cell bodies, whilst the Schwann cells (SCs) ensheath and myelinate single or multiple axon fibres, and finally the macrophages are present for the immune response.
The peripheral nervous system is susceptible to damage due to its limited protection and thus requires the ability to regenerate after damage. However, the mechanism is slow and the results are often unsatisfactory (Scheib and Höke, 2013). The impact of a peripheral nerve injury (PNI)can be detrimental to numerous body functions due to the vast network and different types of nerves that exist in the peripheral nervous system. Injuries to the motor neurons cause muscle weakness (Campbell, 2008), muscle atrophy (Kraft, 1990) and reduced coordination of the muscle, whilst sensory neuronal injuries can lead to the inability to sense touch or increased neuropathic pain (Seltzer et al., 1990; Kim and Chung, 1992), and autonomic nerve damage can lead to organ function abnormalities (Kandel et al., 2000). The development of chronic pain, hypoesthesia and increased pain sensitivity is well known to occur following a PNI, and the mechanisms of nociception following PNI have been recently reviewed (Attal and Bouhassira, 1999; Osborne et al., 2018). A PNI can be caused by overstretching, compression or in some cases, completely or partially severing of the axon (Goubier and Teboul, 2015). Prime examples of causes are dislocations or broken bones following sport- or vehicle-related accidents. The Seddon classification of peripheral nerve injuries divides the damage in to three classes: neuropraxia, azonotmesis and neurotmesis (Seddon, 1942). The Seddon classification has also been expanded by Sunderland in to five degrees of PNI (Mydlarz and Boahene, 2013).
The degree of recovery of sensory neurons following PNI can be determined by the level of cell death within the DRG (Hart et al., 2008). In vivo research using adult rats stated that by two months, up to 35–40% of DRG neurons undergo apoptosis after peripheral injury; and with higher levels of cell death comes reduced functional recovery (McKay Hart et al., 2002). In addition to the high level of cell death, the damage to the nerve results in an increase in inflammation factors. These factors are both beneficial and detrimental to the recovery and hence need to be carefully balanced.
This review will focus on the process that occurs following a PNI and how the altered gene expression within the DRG following a PNI contributes to the inflammation, cellular changes, cell death and subsequent nociception caused by the damage.
The articles reviewed in this manuscript were retrieved by electronic search on the Medline database for literature focused on the gene expression changes in the dorsal root ganglia following peripheral nerve injuries. The following terms were searched: “peripheral nerve injury” AND “dorsal root ganglia” AND “gene expression”. Additionally, the following keywords were used to retrieve further literature: “cell death”, “inflammation” and “nociception”. All the searches results were manually screened for relevance by reading the titles and abstracts. Finally, further searches were performed for specific genes and gene families (e.g., nuclear factor κB (NF-κB), tumor necrosis factor α (TNF-α), caspases, interleukins, neuropeptides).
Pathophysiology of peripheral nerve injury
Processes following peripheral nerve injury
Following PNI, the distal section of the axon undergoes Wallerian degeneration, a process of axon beading, demyelination and fragmentation, finally resulting in the clearance of the debris by macrophages and Schwann cells (SCs) (Hilliard, 2009). The Wallerian degeneration is triggered by the increase in axoplasmic calcium following the axonal damage (George et al., 1995). This process produces an environment which supports the regeneration of the proximal section of the nerve and hence is beneficial for functional recovery (Rotshenker, 2011). Animal models that prevent or delay the process of Wallerian degeneration, such as the C57BL/Ola mice which carry a mutant form of the gene required for Wallerian degeneration (Lyon et al., 1993), have shown impaired neuronal regeneration (Bisby and Chen, 1990). Whilst the distal section of the neuron degrades, the proximal section begins the process of axonal regeneration via the formation of a growth cone (Dahlin and Brandt, 2004).
The primary action following damage to the axon is retrograde signalling whereby a signal is sent from the injury site to the cell body within the DRG (Scheib and Höke, 2013). This process is required to initiate transcriptional changes in the nucleus to increase the production of growth and survival factors to aid neuron regeneration (Abe and Cavalli, 2008). However, the retrograde signalling also contributes to the altered gene expression of factors that contribute to inflammation, cell death and nociception (Li et al., 2015a; Chandran et al., 2016).
The dorsal root ganglia
The DRG consists of the cell bodies of the peripheral sensory neurons; including the large myelinated Aα (Ia and Ib) and Aβ fibres, and small Aδ and C fibres which have little or no myelination respectively (Kandel et al., 2000). Surrounding the neurons there are satellite glial cells (SGCs) which provide trophic support to for the neurons and have been extensively studied (Hanani, 2010), plus a lower abundance of SCs and immune cells such as macrophages (Nascimento et al., 2008; Verkhratsky and Butt, 2013) (Figure 1). It is recognised that both neurones and glial cells can have different contributions to the processes elicited following nerve injury, this review will focus on overall changes in the whole ganglia, rather than on specific cell types.
Glial cells within the dorsal root ganglia
The glial cells within the DRG which surround the neurons are essential for neuronal survival due to their metabolic and structural support (Nascimento et al., 2008). Additional to the changes seen in the damaged neurons, the surrounding cells within the DRG also undergo altered gene expression which leads to phenotypic changes of the cells. The alterations are driven by the novel neuron-glia communication produced by the damaged neurons, and thus are deemed to be a consequence to the changes observed in the neurons (Ohara et al., 2009).
SGCs provide vital support to the neurons under normal conditions and are involved in homeostasis and the immune response (Nascimento et al., 2008). The SGCs are known to proliferate after PNI (Lu and Richardson, 1991) and can be stimulated to change their phenotype and proliferate in vitro by the chemicals released from the damaged neurons such as adenosine triphosphate, TNF-α and nitrous oxide (Verkhratsky and Butt, 2013; Hanstein et al., 2016). Along with an increase in numbers, the SGCs are triggered to increase their production of inflammatory cytokines and neurotrophic factors. Therefore, the SGCs are central to the inflammatory process, and hence, are also involved in the development of nociception and increased cell death (Verkhratsky and Butt, 2013).
In addition to the SGC’s, the SCs are also located in the DRG. The primary role of the SCs is to myelinate the axons to improve conduction velocities of the action potentials. The SCs can be triggered by a PNI to change to a regenerative morphology, increase the production of growth factors (Cheng and Zochodne, 2002) and are important for the regulation of the gene expression within sensory neurons following injury (Poplawski et al., 2018). The knockout of SCs in mice has been shown to induce an increase in the expression of factors required for nerve regeneration both pre- and post-sciatic nerve compression. However, the regeneration in SC lacking mice showed abnormal morphology and the development of neuropathic pain, and highlights the complex role of the SCs in nerve injuries. In addition, as the SCs are primarily located outside of the DRG as axonal sheaths, there are few reports of the specific changes that occur post-injury in the SCs that are located in the DRG.
The macrophages located in the DRG are activated following a PNI. A rat model of sciatic nerve injury reported a 10-fold increase in the DRG after a one week survival which persisted 28 days after the injury (Kwon et al., 2013). The gene expression of neuroprotective and inflammatory cytokines are closely correlated with macrophage activation and will be discussed in further detail within this review.
Therefore, the success of the neuronal regeneration and functional recovery is heavily dependent on the support network of glial cells within the DRG. Hence, to better understand the changes in the gene expression within the cells of the DRG may enable the development of novel therapeutic targets to enhance the recovery process.
Gene Expression Changes in the Dorsal Root Ganglia Following PNI
The gene expression within the DRG is dynamic, and can respond to a damage, be it a sudden injury or a chronic compression. The cells need to be able to alter the gene expression, and subsequently alter the level of protein production of certain factors to respond to the damage. In addition to the pro-survival and regeneration factors, the altered gene expression also contributes to inflammation, cell death and nociception. This section will highlight the studies which have investigated the gene expression of the main contributors of these detrimental processes within the DRG (Figure 2).
Figure 2.
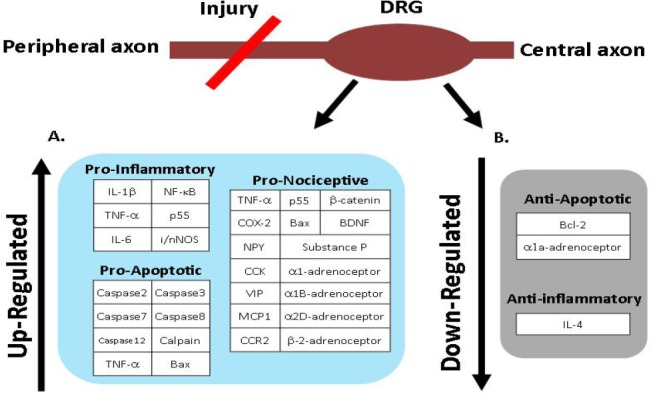
Summary diagram of the gene expression changes within the dorsal root ganglia (DRG) which occur post-injury to the peripheral nerve.
The factors have been separated by effect, and up- (A) or down-regulation (B). IL-1β: Interleukin-1β; NF-κB: necrosis factor κB; TNF-α: tumour necrosis factor α; p55: TNF-α receptor; IL-6: interleukin-6; i/nNOS: inducible/neuronal nitric oxide synthase; Bax: Bcl-2-associated X protein; COX-2: cyclooxygenase 2; BDNF: brain derived neurotrophic factor; NPY: neuropeptide Y; CCK: cholecytokinin; VIP: vasoactive intestinal peptide; MCP: monocyte chemoattractant protein; CCR2: C-C chemokine receptor type 2; Bcl-2: B-cell lymphoma 2; IL-4: interleukin-4.
How altered gene expression within the dorsal root ganglia contributes to inflammation
Firstly, it is important to state that inflammation is a complex and vast topic, with both beneficial and detrimental properties following a PNI. A detailed review of the role of inflammation following PNI can be found by Fregnan et al. (2012). Whilst acute inflammation is required to clear the cellular debris and improve the environment for regeneration, the development of chronic inflammation results in cellular death and nociception, and hence is detrimental to recovery (Rock, 2009). The presence of chronic inflammation has shown to cause a down-regulation of the growth associated phosphoprotein 43 within DRG neurons (Kato et al., 2003). The role of the inflammatory regulators are not restricted to inflammation and TNF-α is associated with other pathways including necrosis (Bradley, 2008), and inducing increased excitability of DRG neurons associated with nociceptive pain (Zhang et al., 2007). Also, the increase in pro-inflammatory mediators can lead to altered signalling within the spinal cord including synaptic reorganisation and sensitisation which can produce a heightened sensation of pain (Latremoliere and Woolf, 2009). Therefore, an understanding of the regulation of inflammatory mediators may help to reduce cell death and prevent neuropathic pain following a PNI.
Here we will outline the changes in the gene expression of inflammatory cytokines. The inflammatory response within the DRG following PNIs is mediated by SGCs, SCs, macrophages and lymphocytes, with unique temporal and spatial characteristic for each cell type. The prominent regulators of the pro-inflammatory response are NF-κB, TNF-α, interleukin (IL)-1β and IL-6.
Nuclear factor κB
NF-κB is a protein complex that activates signalling pathways which result in the production of cytokines, cell survival and the regulation of transcription (Lawrence, 2009). Within unstimulated cells, NF-κB is found in an inactive form bound to the inhibitor IκB, therefore, it is ubiquitously present in cells and upregulated if the normal level is not sufficient for the response (Chen et al., 1999). The NF-κB pathway triggers the production of cytokines including IL-1β and TNF-α (Lawrence, 2009); which increases the immune cell response and can increase the transcription of NF-κB itself.
Tumor necrosis factor α
TNF-α is central to the pro-inflammatory response following a PNI and is produced by the glial cells, SGCs and SCs, and immune cells such as macrophages. The increase in TNF-α has a causal effect to increase the activation of pro-inflammatory pathways including NF-κB and inducible nitric oxide synthase.
An investigation of a sciatic nerve crush injury in mice used immunohistochemistry to conclude that both TNF-α and its receptor (p55) were increased in the SGCs within the DRG. In contrast, the receptor p55 was only present in the DRG neurons after injury (Ohtori et al., 2004).
An inflammation model using systematic challenge with lipopolysaccharide investigated the altered expression of TNF-α in the DRG (Schäfers et al., 2002). Whilst the study did not use a nerve injury model, the study highlights the altered expression level of TNF-α within an inflammatory environment. Real-time PCR and immunohistochemistry concluded that 1 hour post lipopolysaccharide treatment, TNF-α increased within the non-neuronal cells and not within the neuronal cells of the DRG. The TNF-α receptors were also studied using western blotting and real-time PCR and results showed that TNF-α receptor 1 expression increased 2.5-fold and peaked at 6 hours after lipopolysaccharide challenge, whilst TNF-α receptor 2 peaked at a 2.7-fold increase at 3 hours and reduced to control levels by 6 hours (Schäfers et al., 2002).
The time-point of the upregulation of TNF-α was investigated by Sacerdote et al. (2008) and Üçeyler et al. (2007) in a mouse model of a chronic constriction injury. Both studies analysed the changes in the gene expression within the DRG via real-time quantitative PCR. The study by Üçeyler et al. (2007) studied the gene expression changes within the DRG at six time-points within 24 hours. They reported a downregulation of TNF-α mRNA within the ipsilateral and contralateral DRG at 1 hour post injury, and a subsequent 1.8-fold increase at the 24-hour time-point. Furthermore, Sacerdote et al. (2008) investigated a wider time window and reported that TNF-α mRNA expression increased 2.5-fold on days one and three after surgery, and that the expression level reduced by days seven and fourteen and was equivalent to the sham-surgery group.
In addition, a study by Abe et al. (2003), reported an increase in TNF-α in the ipsilateral DRG following a sciatic tourniquet. The upregulation peaked at 1 hour post application of the tourniquet, and gradually decreased at the 2-hour and 4-hour time points (Abe et al., 2003).
Furthermore, Dubový et al. (2006) also investigated the increase in TNF-α and the receptor (p55) proteins following a unilateral sciatic nerve ligation or transection. The ipsilateral and contralateral DRG was investigated by immunofluorescence. They discovered that the neurons and glial cells respond differently to the two types of injury of the sciatic nerve; either ligation or transection. Firstly, the ligation of the sciatic nerve resulted in the increased abundance of TNF-α within the contralateral DRG neurons for up to 2 weeks, whilst the SGCs displayed a bilateral increase. They also reported an increase in the infiltration of TNF-α positive macrophages surrounding the DRG neuron cell bodies. The ligation also caused a bilateral increase of the TNF-α receptor, p55, in the DRG neurons and SGCs, with a higher abundance at 2 weeks than 1 week. In comparison, following nerve transection, the DRG neurons and SGC both showed bilateral increase in TNF-α (Dubový et al., 2006).
Interleukin family
The presence of IL-1β is found within macrophages, large and medium sensory neurons, yet only sporadically in small-diameter sensory neurons (Copray et al., 2001). The expression of IL-1β mRNA is found in approximately 70% of DRG neurons, with the production of the IL-1β protein being found in 80% of these neurons. The IL-1β receptor is located in DRG neurons and glial cells (presumed to be SGCs) (Copray et al., 2001).
The expression of IL-1β was investigated in a mouse model using a chronic constriction injury of the sciatic nerve (Uçeyler et al., 2007). Real-time PCR was used and reported a phasic change in the expression of IL-1β mRNA within the ipsilateral DRG during a 24-hour time window. Following an initial decrease in expression at 1 hour post injury, the expression of IL-1β increased at both 6- and 12-hour time-points. The contralateral DRG also showed the increase at 6 and 12 hour time-points, however, in contrast, there was also an increase in expression at the 1 hour time-point.
The study by Üçeyler et al. (2007) also investigated the anti-inflammatory cytokines IL-4 and IL-10. The IL-4 mRNA expression was shown to reduce at 3 hours post injury only within the ipsilateral DRG. In contrast, IL-10 showed a significant increase in the expression, yet this was only at the 24-hour time-point. This would indicate a strong initial increase in inflammation within the DRG due to the reduced levels of the anti-inflammatory interleukins following the PNI.
Furthermore, the expression level of IL-6, an interleukin involved in both pro- and anti-inflammation, was also reported by Murphy et al. (1995) to increase following a sciatic nerve injury in rats. As a control, real-time PCR was carried out on the contralateral (to injury) and un-operated rats DRG, and reported no detectable IL-6 mRNA. However, post-injury, the ipsilateral DRG reported an increase from one day ( first measurement post-injury). The increased level of IL-6 mRNA was seen to peak at two and four days, and measurements on day eight and fourteen reported no detectable IL-6 mRNA (Murphy et al., 1995). A more recent study by Vega-Avelaira et al. (2009), measured IL-6 expression with real-time PCR at seven days post-sciatic nerve injury and also measured an increased expression within the DRG. The results were seen in young and old rats in comparison to sham-operated rats.
Nitric oxide synthase
A prominent marker for the presence of macrophages is inducible nitric oxide synthase and thus an upregulation of gene expression is correlated with the infiltration of macrophages and an increase in the inflammatory response. A study which applied real-time PCR to investigate the gene expression of inducible nitric oxide synthase and neuronal nitric oxide synthase within a rat DRG following a peripheral tourniquet, demonstrated that the mRNA and protein levels of inducible nitric oxide synthase and neuronal nitric oxide synthase increase in the ipsilateral DRG, yet not within the contralateral or control DRGs (Abe et al., 2003). The high degree of inducible nitric oxide synthase within the DRG provides further evidence of an increased inflammatory response which subsequently could induce apoptosis of the surrounding cells.
How altered gene expression within the dorsal root ganglia contributes to cell death
Transection of the sciatic nerve induces the death of DRG neurons which is detrimental to functional recovery; with rodent studies reporting 6–35% loss depending on the length of survival after injury (Groves et al., 1997; Vestergaard et al., 1997; Shi et al., 2001). A study by Whiteside (1998) concluded that cell death within the DRG was predominantly neuronal up to 3 days after PNI, whilst subsequent cell death primarily occurs in the glial cells. This is likely to be due to the glial cells dependence on the neurons for their survival. Equally, the surrounding glial cells within the DRG are vital for functional recovery of the axons and their death is thus detrimental to the recovery of the nerve (Vigneswara et al., 2013).
Caspase family
The caspase family is well established to play a central role in apoptosis following damage to cells. The PNI causes an altered expression of caspase enzymes within the DRG, and increases the abundance of pro-apoptotic factors (Momeni et al., 2013; Vigneswara et al., 2013).
Firstly, caspase-2 was shown to be increased at thirty days after a sciatic nerve transection in DRG neurons and SGCs in rats (Vigneswara et al., 2013). The increase was measured by immunohistochemistry and in comparison to contralateral control DRGs. In contrast they reported that the expression of caspase-3 was shown to reduce in DRG neurons and SGCs (Vigneswara et al., 2013). Interestingly, the silencing of caspase-2 via small interfering RNA-mediated knockdown of the caspase-2 gene protected the neurons from apoptosis after serum withdrawal. In comparison, the silencing of caspase-3 did not result in a reduction in apoptosis and implies that caspase-2 has a larger role in the apoptotic process (Vigneswara et al., 2013).
However, an opposite conclusion was drawn by Vega-Avelaira et al. (2009) and Wiberg et al. (2018) such that quantitative real-time PCR showed an increase in caspase-3 within the DRG. Vega-Avelaira et al. (2009) demonstrated that there the increase occurred at 7 days post sciatic nerve injury and was seen in both young and old rats. Furthermore, Wiberg et al. (2018) also reported an increase in caspase-3 at 7-, 14- and 28-day post nerve transection.
The difference in the time of measurement, plus differing measurement techniques between studies could explain the difference seen in Vigneswara et al. (2013), Wiberg et al. (2018), and Vega-Avelaira et al. (2009).
The expression of caspase-8, caspase-7 and caspase-12 were also reported to be upregulated at 7, 14, and 28 days following a peripheral nerve transection (Wiberg et al., 2018). The upregulation of caspase-8 and caspase-7 peaked at day 14, whilst caspase-12 peaked at 7 days post injury and was associated with the upregulation of its activator Calpain (Wiberg et al., 2018). They concluded that the increased expression of these factors induced the endoplasmic reticulum stress mediated apoptosis pathway which is common within a subtype of DRG neurons, and may explain the high degree of neuronal cell death within the DRG post PNI.
Tumor necrosis factor α
TNF-α has previously been mentioned in regards to its role in inflammation, however, it is not its only role and is also involved in cell death following PNI. TNF-α is more abundant following PNI, which subsequently induces an increase in the cell-death signalling cascade. For instance, the upregulation of TNF-α increases the activation of the pro-apoptosis factor, caspase-8 (Micheau and Tschopp, 2003).
B-cell lymphoma 2 gene family
The B-cell lymphoma 2 (Bcl-2) family of apoptosis regulators have also been investigated in their contribution to cell death within the DRG after PNI. Additional to the upregulation of the pro-apoptotic factors, there is also the reduced expression of the anti-apoptotic factors. A study by Gillardon et al. (1996) used PCR to measure the expression of the anti-apoptosis factor Bcl-2 in the DRG of young and adult rats after 5 days after a unilateral transection of the sciatic nerve. In both age groups the expression of Bcl-2 mRNA was down-regulated. They also reported that the young rats had a significantly greater reduction in Bcl-2 expression within the DRG compared to the adult rats. Further measurements taken at 20 days highlighted a partial recovery within both groups.
In contrast, the pro-apoptotic factor Bax, did not show a significant difference up to twenty days post-sciatic nerve injury, nor any difference was noted between young and old rats (Gillardon et al., 1996). The reduction in the protective Bcl-2 gene following PNI and the ubiquitously high expression of the pro-apoptosis factor Bax, hence results in an increased Bax/Bcl-2 ratio. Thus, this change in ratio explains the increase in apoptosis within the DRG following a PNI.
To the best of our knowledge, there are no reports of the Bcl-2 gene family cell death promotor factors Bid, Bak and Bad. Nevertheless, they are part of the pro-apoptotic signalling pathway and therefore, it is likely that these factors also increase in expression. Furthermore, there are no studies reporting the expression changes of the caspase-independent apoptotic pathway via the apoptosis inducing factor.
How altered gene expression within the dorsal root ganglia contributes to nociceptive transmission
The development of pain sensitivity and chronic pain is common after a PNI. The initial protective role of pain to prevent further damage can progress to become an unwanted chronic pain due to the persistent upregulation of specific channels, receptors and signalling molecules. The increase of inflammation and cell death can also increase the risk of the development of neuropathic pain. This section will highlight prominent genes that result in the altered pain signalling and unwanted neuropathic pain.
Tumor necrosis factor α
As previously mentioned, TNF-α and its receptor increases in the DRG following PNI (Ohtori et al., 2004). The increased level of TNF-α signalling increases the excitability of the DRG neurons, lowering the threshold required for activation of the nociceptive neurons and thus contributes to the persistent pain experienced after injury (Vogel et al., 2006). The study by Sacerdote et al. (2008) previously discussed in relation to TNF-α’s role in inflammation used a neuropathic pain model by chronically constricting the sciatic nerve. They reported increased TNF-α mRNA levels in the DRG maintained from day one to day three post-surgery (Sacerdote et al., 2008). Alongside the increased cytokine levels, the injured mice displayed mechanical allodynia and hyperalgesia from six hours after surgery, with mechanical allodynia persisting until day 14. However, it is important to note that the sham-operated mice also displayed an initial increase of TNF-α at 6 hours after surgery, nevertheless unlike the injured group the rise in TNF-α was not maintained. This suggests that the maintained increase in TNF-α signalling results in the sensitization and an increase of pain transmission (Sacerdote et al., 2008).
The specific location of the changes in TNF-α expression which contributes to heightened nociceptive transmission has been investigated by Schäfers et al. (2003). The ipsilateral DRGs were removed four days after ligation to the sciatic nerve. The expression of TNF-α after chronic constriction injury was higher, specifically in the medium-sized neurons and were co-localised with tropomyosin receptor kinase B and neurofilament (Schäfers et al., 2003). The changes in the level of TNF-α signalling have been considered to contribute to an altered phenotype of the low-threshold primary afferents which lowers their threshold for activation. In addition, the study also highlighted that the uninjured DRG neurons adjacent to injured neurons also showed an increased abundance of TNF-α. This is regarded to be due to the exposure to the hostile environment caused by the increased cytokines and growth factors.
Neuropeptides
The damage to the DRG neurons triggers a change in the expression of neuropeptides which are involved in the communication between cells and the signalling of pain. Numerous neuropeptides are upregulated and are linked with the increase in neuropathic pain; however, their role is not restricted to nociception and some also have roles in survival and regeneration. For example, Substance P and calcitonin gene-related peptide are neuropeptides that show a transient upregulation in the DRG after a sciatic nerve injury and can potentially be used for evaluating the early stages of injury (Fu et al., 2013). The increase in expression was measured using immunohistochemical techniques and peaked at day seven, and showed a reduction at fourteen days and the return to baseline by 28 days post-sciatic nerve injury. The main roles of Substance P and calcitonin gene-related peptide is in pain transmission, however, downstream targets associate them with promoting neuronal regeneration, highlighting that they have both beneficial and detrimental outcomes (Fu et al., 2013).
The neuropeptide Y, galanin and cholecystokinin are associated with allodynia following PNI in rats (Shi et al., 1999) and are shown to increase ten-fold at 2, 7, 14, and 28 days post-PNI (Xiao et al., 2002). However, there are beneficial outcome in regeneration following an increase in neuropeptide Y (Terenghi, 1999), galanin (Xu et al., 2016) and cholecystokinin (Zhang et al, 1993), and hence theses neuropeptides would not make them likely to be good targets to treat PNI induced pain.
Brain-derived neurotrophic factor
The neurotrophic factor brain-derived neurotrophic factor (BDNF) also has dual roles and along with promoting regeneration, it has been shown to contribute to the increased nociceptive transmission subsequent to inflammation (Obata and Noguchi, 2006). BDNF has been shown to increase in expression within the DRG neurons (Ha et al., 2001). The increase in BDNF expression occurs within the small, medium and large neurons and has shown to be injury dependent. The chronic constriction of the sciatic nerve resulted in an increase in BDNF within all neuron sizes at seven and fourteen days post-surgery (Ha et al., 2001). On the other hand, seven days after the ligation of the sciatic nerve resulted in an increased BDNF expression only within the medium and large neurons of the DRG.
Uchida et al. (2013) have investigated the role of BDNF as a therapeutic target to decrease neuropathic pain following a PNI. In a compelling study they used a sciatic nerve injury mice model and reported that the administering of an anti-BDNF antibody inhibited thermal hyperalgesia and mechanical allodynia (Uchida et al., 2013).
Chemokines and chemokine receptors
There are numerous molecules that are involved in the regulation of pain including chemokines and their receptors. For example, monocyte chemoattractant protein 1 (also known as chemokine C-C ligand) and its receptor C-C chemokine receptor type 2 are upregulated in the DRG after a variety of injury animal models including chronic constriction, sciatic nerve ligation and sciatic nerve transection (Tanaka et al., 2004; Jeon et al., 2009). The chemokine is involved in the immune response by increasing monocytes in the affected area. However, monocyte chemoattractant protein 1 has been linked to the development of enhanced excitement of nociceptive DRG neurons which lowers the threshold for activation and hence, increased pain transmission (Abbadie, 2005; White et al., 2007). The administration of monocyte chemoattractant protein 1 to small, medium and large DRG neurons causes depolarisation and action potentials within animal models of chronic compression of the DRG (Sun et al., 2006).
The C-C chemokine receptor type 2 receptors are not located in naïve DRG neurons and investigations have shown that chronic constriction of the DRG results in an increase in their abundance. Furthermore, a transgenic mice model, overexpressing monocyte chemoattractant protein 1 within glial cells, presented with higher nociceptive responses than controls (Menetski et al., 2007). Hence, monocyte chemoattractant protein 1 and C-C chemokine receptor type 2 are deemed to contribute to nociceptive pain transmission and highlights the central role of glial cells in nociception transmission.
Adrenergic transmission
Adrenergic receptors (ARs) have been implicated in the development of neuropathic pain as they are activated by catecholinergic molecules, such as noradrenaline, which is involved in the pain response (Pertovaara, 2006). The administration of α-adrenergic receptor antagonists have shown to have an anti-nociceptive effect (Stone et al., 1999). The expression of different ARs within the DRG is altered following a PNI (Drummond, 2014).
A study using RNase protection assay and in situ hybridisation used a PNI rat model to investigate the expression of the α1-AR subtypes; α1a-AR, α1b-AR, and α1d-AR (Xie et al., 2001). Firstly, in controls DRGs, there is a moderate abundance of α1a-AR, and a low abundance of α1b-AR and α1d-AR. The RNase protection assay measured the mRNA level at seven days post-PNI, and showed a 21% reduction in α1a-AR, a 39% increase in α1b-AR and no difference in the α1d-AR. Further in situ hybridisation analysis of the α1b-AR showed that expression increased from 7.9% of neurons, to 20.4% after PNI (Xie et al., 2001).
In addition, the α2-A-adrenoceptors have been shown to increase 5-fold in the DRG neurons following sciatic nerve transection; with the highest degree of change seen in the medium neurons with low threshold mechanoreceptors (Birder and Perl, 1999). Petersen et al. (1996) demonstrated that a higher ratio of DRG neurons responded to noradrenaline eleven to twenty five days after nerve injury, and hence increased nociceptive transmission, which supports the evidence of up-regulation of the adrenergic receptors contributing to pain.
Cyclooxygenase 2
The enzyme cyclooxygenase 2 is pivotal in the expression of Substance P, a peptide involved in nociceptive transmission. A study by Li et al. (2015b) demonstrated how a chronic constriction injury within rats induced the upregulation of β-catenin, the activator of cyclooxygenase 2, within the ipsilateral DRG. The increase in gene expression was correlated with the upregulation of Substance P gene expression and protein release. The importance of cyclooxygenase 2 in the production of Substance P was highlighted by the successful reduction in Substance P by inhibiting the cyclooxygenase 2 induction (Li et al., 2015b).
Conclusion
In conclusion, this review has highlighted how the expression of a variety of molecules within the DRG is dynamic and injury-dependent which leads to the hostile environment post PNI. We have also highlighted how the different cell types act differently to one another and contribute to inflammation and nociceptive transmission, in addition to the locations of where cell death occurs post-injury. The multitude of changes at the expression level within each cell type, and the injury-type specific responses that we have highlighted in this review, emphasise the complexity of the response to injury. Further research will hopefully progress to a therapeutic treatment to enhance the regeneration and survival of the neurons, in addition to preventing the development of abnormal nociceptive pain transmission.
Footnotes
Conflicts of interest: None declared.
Financial support: The work was supported by the Hargreaves and Ball Trust, the National Institute for Health Research (II-LA-0313-20003) (to AJR), the Rosetrees Trust, the Academy of Medical Sciences, and the Manchester Regenerative Medicine Network (MaRMN) (to AF and AJR), Progetto Eccellenza from the Italian Ministry of Research (to VM).
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Funding: The work was supported by the Hargreaves and Ball Trust, the National Institute for Health Research (II-LA-0313-20003) (to AJR), the Rosetrees Trust, the Academy of Medical Sciences, and the Manchester Regenerative Medicine Network (MaRMN) (to AF and AJR), Progetto Eccellenza from the Italian Ministry of Research (to VM).
C-Editors: Zhao M, Yu J; T-Editor: Liu XL
References
- 1.Abbadie C. Chemokines, chemokine receptors and pain. Trends Immunol. 2005;26:529–534. doi: 10.1016/j.it.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 2.Abe N, Cavalli V. Nerve injury signaling. Curr Opin Neurobiol. 2008;18:276–283. doi: 10.1016/j.conb.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abe S, Mizusawa I, Kanno K, Yabashi A, Suto M, Kuraya M, Honda T, Hiraiwa K. Nitric oxide synthase expressions in rat dorsal root ganglion after a hind limb tourniquet. Neuroreport. 2003;14:2267–2270. doi: 10.1097/00001756-200312020-00026. [DOI] [PubMed] [Google Scholar]
- 4.Attal N, Bouhassira D. Mechanisms of pain in peripheral neuropathy. Acta Neurol Scand. 1999;Suppl 173:12–24. doi: 10.1111/j.1600-0404.1999.tb07386.x. discussion 48-52. [DOI] [PubMed] [Google Scholar]
- 5.Birder LA, Perl ER. Expression of alpha2-adrenergic receptors in rat primary afferent neurones after peripheral nerve injury or inflammation. J Physiol. 1999;515:533–542. doi: 10.1111/j.1469-7793.1999.533ac.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bisby MA, Chen S. Delayed wallerian degeneration in sciatic nerves of C57BL/Ola mice is associated with impaired regeneration of sensory axons. Brain Res. 1990;530:117–120. doi: 10.1016/0006-8993(90)90666-y. [DOI] [PubMed] [Google Scholar]
- 7.Bradley JR. TNF-mediated inflammatory disease. J Pathol. 2008;214:149–160. doi: 10.1002/path.2287. [DOI] [PubMed] [Google Scholar]
- 8.Campbell WW. Evaluation and management of peripheral nerve injury. Clin Neurophysiol. 2008;119:1951–1965. doi: 10.1016/j.clinph.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 9.Chandran V, Coppola G, Nawabi H, Omura T, Versano R, Huebner EA, Zhang A, Costigan M, Yekkirala A, Barrett L, Blesch A, Michaelevski I, Davis-Turak J, Gao F, Langfelder P, Horvath S, He Z, Benowitz L, Fainzilber M, Tuszynski M, et al. A Systems-level analysis of the peripheral nerve intrinsic axonal growth program. Neuron. 2016;89:956–970. doi: 10.1016/j.neuron.2016.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen F, Castranova V, Shi X, Demers LM. New insights into the role of nuclear factor-kappaB, a ubiquitous transcription factor in the initiation of diseases. Clin Chem. 1999;45:7–17. [PubMed] [Google Scholar]
- 11.Cheng C, Zochodne DW. In vivo proliferation, migration and phenotypic changes of Schwann cells in the presence of myelinated fibers. Neuroscience. 2002;115:321–329. doi: 10.1016/s0306-4522(02)00291-9. [DOI] [PubMed] [Google Scholar]
- 12.Copray JC, Mantingh I, Brouwer N, Biber K, Kust BM, Liem RS, Huitinga I, Tilders FJ, Van Dam AM, Boddeke HW. Expression of interleukin-1 beta in rat dorsal root ganglia. J Neuroimmunol. 2001;118:203–211. doi: 10.1016/s0165-5728(01)00324-1. [DOI] [PubMed] [Google Scholar]
- 13.Dahlin LB, Brandt J. Basic science of peripheral nerve repair: Wallerian degeneration/growth cones. Oper Tech Orthop. 2004;14:138–145. [Google Scholar]
- 14.Drummond PD. Neuronal changes resulting in up-regulation of alpha-1 adrenoceptors after peripheral nerve injury. Neural Regen Res. 2014;9:1337–1340. doi: 10.4103/1673-5374.137583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dubový P, Jančálek R, Klusáková I, Svíženská I, Pejchalová K. Intra- and extraneuronal changes of immunofluorescence staining for TNF-alpha and TNFR1 in the dorsal root ganglia of rat peripheral neuropathic pain models. Cell Mol Neurobiol. 2006;26:1203–1215. doi: 10.1007/s10571-006-9006-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fregnan F, Muratori L, Simoes AR, Giacobini-Robecchi MG, Raimondo S. Role of inflammatory cytokines in peripheral nerve injury. Neural Regen Res. 2012;7:2259–2266. doi: 10.3969/j.issn.1673-5374.2012.29.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fu C, Yin Z, Yu D, Yang Z. Substance P and calcitonin gene-related peptide expression in dorsal root ganglia in sciatic nerve injury rats. Neural Regen Res. 2013;8:3124–3130. doi: 10.3969/j.issn.1673-5374.2013.33.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.George EB, Glass JD, Griffin JW. Axotomy-induced axonal degeneration is mediated by calcium influx through ion-specific channels. J Neurosci. 1995;15:6445–6452. doi: 10.1523/JNEUROSCI.15-10-06445.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gillardon F, Klimaschewski L, Wickert H, Krajewski S, Reed JC, Zimmermann M. Expression pattern of candidate cell death effector proteins Bax, Bcl-2, Bcl-X, and c-Jun in sensory and motor neurons following sciatic nerve transection in the rat. Brain Res. 1996;739:244–250. doi: 10.1016/s0006-8993(96)00829-3. [DOI] [PubMed] [Google Scholar]
- 20.Goubier JN, Teboul F. Chapter 38 - grading of nerve injuries. In: Nerves and Nerve Injuries. In: Tubbs RS, Rizk E, Shoja MM, Loukas M, Barbaro N, Spinner RJ, editors. San Diego: Academic Press; 2015. pp. 603–610. [Google Scholar]
- 21.Groves MJ, Christopherson T, Giometto B, Scaravilli F. Axotomy-induced apoptosis in adult rat primary sensory neurons. J Neurocytol. 1997;26:615–624. doi: 10.1023/a:1018541726460. [DOI] [PubMed] [Google Scholar]
- 22.Ha SO, Kim JK, Hong HS, Kim DS, Cho HJ. Expression of brain-derived neurotrophic factor in rat dorsal root ganglia, spinal cord and gracile nuclei in experimental models of neuropathic pain. Neuroscience. 2001;107:301–309. doi: 10.1016/s0306-4522(01)00353-0. [DOI] [PubMed] [Google Scholar]
- 23.Hanani M. Satellite glial cells in sympathetic and parasympathetic ganglia: in search of function. Brain Res Rev. 2010;64:304–327. doi: 10.1016/j.brainresrev.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 24.Hanstein R, Hanani M, Scemes E, Spray DC. Glial pannexin1 contributes to tactile hypersensitivity in a mouse model of orofacial pain. Sci Rep. 2016;6:38266. doi: 10.1038/srep38266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hart AM, Terenghi G, Wiberg M. Neuronal death after peripheral nerve injury and experimental strategies for neuroprotection. Neurol Res. 2008;30:999–1011. doi: 10.1179/174313208X362479. [DOI] [PubMed] [Google Scholar]
- 26.Hilliard MA. Axonal degeneration and regeneration: a mechanistic tug-of-war. J Neurochem. 2009;108:23–32. doi: 10.1111/j.1471-4159.2008.05754.x. [DOI] [PubMed] [Google Scholar]
- 27.Huebner EA, Strittmatter SM. Axon regeneration in the peripheral and central nervous systems. Results Probl Cell Differ. 2009;48:339–351. doi: 10.1007/400_2009_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jeon SM, Lee KM, Cho HJ. Expression of monocyte chemoattractant protein-1 in rat dorsal root ganglia and spinal cord in experimental models of neuropathic pain. Brain Res. 2009;1251:103–111. doi: 10.1016/j.brainres.2008.11.046. [DOI] [PubMed] [Google Scholar]
- 29.Kandel ER, Schwartz JH, Jessel TM. 4th ed. New York: McGraw-Hill Companies; 2000. Principles of Neural Science. [Google Scholar]
- 30.Kato N, Nemoto K, Arino H, Fujikawa K. Influence of peripheral inflammation on growth-associated phosphoprotein (GAP-43) expression in dorsal root ganglia and on nerve recovery after crush injury. Neurosci Res. 2003;45:297–303. doi: 10.1016/s0168-0102(02)00234-1. [DOI] [PubMed] [Google Scholar]
- 31.Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–363. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- 32.Kraft GH. Fibrillation potential amplitude and muscle atrophy following peripheral nerve injury. Muscle Nerve. 1990;13:814–821. doi: 10.1002/mus.880130907. [DOI] [PubMed] [Google Scholar]
- 33.Kwon MJ, Kim J, Shin H, Jeong SR, Kang YM, Choi JY, Hwang DH, Kim BG. Contribution of macrophages to enhanced regenerative capacity of dorsal root ganglia sensory neurons by conditioning injury. J Neurosci. 2013;33:15095–15108. doi: 10.1523/JNEUROSCI.0278-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10:895–926. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol. 2009;1:a001651. doi: 10.1101/cshperspect.a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li S, Xue C, Yuan Y, Zhang R, Wang Y, Wang Y, Yu B, Liu J, Ding F, Yang Y, Gu X. The transcriptional landscape of dorsal root ganglia after sciatic nerve transection. Sci Rep. 2015a;5:16888. doi: 10.1038/srep16888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li YS, Xi Y, Li XJ, Leng CL, Jia MM, Zhang WK, Tang HB. Up-regulation of the biosynthesis and release of substance P through Wnt/beta-catenin signaling pathway in rat dorsal root ganglion cells. PLoS One. 2015b;10:e0129701. doi: 10.1371/journal.pone.0129701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu X, Richardson PM. Inflammation near the nerve cell body enhances axonal regeneration. J Neurosci. 1991;11:972–978. doi: 10.1523/JNEUROSCI.11-04-00972.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lyon MF, Ogunkolade BW, Brown MC, Atherton DJ, Perry VH. A gene affecting Wallerian nerve degeneration maps distally on mouse chromosome 4. Proc Natl Acad Sci U S A. 1993;90:9717–9720. doi: 10.1073/pnas.90.20.9717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McKay Hart A, Brannstrom T, Wiberg M, Terenghi G. Primary sensory neurons and satellite cells after peripheral axotomy in the adult rat: timecourse of cell death and elimination. Exp Brain Res. 2002;142:308–318. doi: 10.1007/s00221-001-0929-0. [DOI] [PubMed] [Google Scholar]
- 41.Menetski J, Mistry S, Lu M, Mudgett JS, Ransohoff RM, Demartino JA, Macintyre DE, Abbadie C. in astrocytes display enhanced nociceptive responses. Neuroscience. 2007) Mice overexpressing chemokine ligand 2 (CCL2;149:706–714. doi: 10.1016/j.neuroscience.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 42.Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114:181–190. doi: 10.1016/s0092-8674(03)00521-x. [DOI] [PubMed] [Google Scholar]
- 43.Momeni HR, Soleimani Mehranjani M, Shariatzadeh MA, Haddadi M. Caspase-mediated apoptosis in sensory neurons of cultured dorsal root Ganglia in adult mouse. Cell J. 2013;15:212–217. [PMC free article] [PubMed] [Google Scholar]
- 44.Murphy PG, Grondin J, Altares M, Richardson PM. Induction of interleukin-6 in axotomized sensory neurons. J Neurosci. 1995;15:5130–5138. doi: 10.1523/JNEUROSCI.15-07-05130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mydlarz WK, Boahene KO. Sunderland classification of nerve injury. In: Encyclopedia of Otolaryngology, Head and Neck Surgery. In: Kountakis SE, editor. Berlin, Heidelberg: Springer Berlin Heidelberg; 2013. pp. 2615–2616. [Google Scholar]
- 46.Nascimento RS, Santiago MF, Marques SA, Allodi S, Martinez AM. Diversity among satellite glial cells in dorsal root ganglia of the rat. Braz J Med Biol Res. 2008;41:1011–1017. doi: 10.1590/s0100-879x2008005000051. [DOI] [PubMed] [Google Scholar]
- 47.Obata K, Noguchi K. BDNF in sensory neurons and chronic pain. Neurosci Res. 2006;55:1–10. doi: 10.1016/j.neures.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 48.Ohara PT, Vit JP, Bhargava A, Romero M, Sundberg C, Charles AC, Jasmin L. Gliopathic pain: when satellite glial cells go bad. Neuroscientist. 2009;15:450–463. doi: 10.1177/1073858409336094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ohtori S, Takahashi K, Moriya H, Myers RR. TNF-alpha and TNF-alpha receptor type 1 upregulation in glia and neurons after peripheral nerve injury: studies in murine DRG and spinal cord. Spine (Phila Pa 1976) 2004;29:1082–1088. doi: 10.1097/00007632-200405150-00006. [DOI] [PubMed] [Google Scholar]
- 50.Osborne NR, Anastakis DJ, Davis KD. Peripheral nerve injuries, pain, and neuroplasticity. J Hand Ther. 2018;31:184–194. doi: 10.1016/j.jht.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 51.Pertovaara A. Noradrenergic pain modulation. Prog Neurobiol. 2006;80:53–83. doi: 10.1016/j.pneurobio.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 52.Petersen M, Zhang J, Zhang JM, LaMotte RH. Abnormal spontaneous activity and responses to norepinephrine in dissociated dorsal root ganglion cells after chronic nerve constriction. Pain. 1996;67:391–397. doi: 10.1016/0304-3959(96)03146-6. [DOI] [PubMed] [Google Scholar]
- 53.Poplawski G, Ishikawa T, Brifault C, Lee-Kubli C, Regestam R, Henry KW, Shiga Y, Kwon H, Ohtori S, Gonias SL, Campana WM. Schwann cells regulate sensory neuron gene expression before and after peripheral nerve injury. Glia. 2018 doi: 10.1002/glia.23325. doi: 10.1002/glia.23325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rock KL. Pathobiology of inflammation to cell death. Biol Blood Marrow Transplant. 2009;15:137–138. doi: 10.1016/j.bbmt.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rotshenker S. Wallerian degeneration: the innate-immune response to traumatic nerve injury. J Neuroinflammation. 2011;8:109. doi: 10.1186/1742-2094-8-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sacerdote P, Franchi S, Trovato AE, Valsecchi AE, Panerai AE, Colleoni M. Transient early expression of TNF-alpha in sciatic nerve and dorsal root ganglia in a mouse model of painful peripheral neuropathy. Neurosci Lett. 2008;436:210–213. doi: 10.1016/j.neulet.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 57.Schäfers M, Svensson CI, Sommer C, Sorkin LS. Tumor necrosis factor-alpha induces mechanical allodynia after spinal nerve ligation by activation of p38 MAPK in primary sensory neurons. J Neurosci. 2003;23:2517–2521. doi: 10.1523/JNEUROSCI.23-07-02517.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schäfers M, Geis C, Brors D, Yaksh TL, Sommer C. Anterograde transport of tumor necrosis factor-alpha in the intact and injured rat sciatic nerve. J Neurosci. 2002;22:536–545. doi: 10.1523/JNEUROSCI.22-02-00536.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Scheib J, Höke A. Advances in peripheral nerve regeneration. Nat Rev Neurol. 2013;9:668–676. doi: 10.1038/nrneurol.2013.227. [DOI] [PubMed] [Google Scholar]
- 60.Seddon HJ. A classification of nerve injuries. Br Med J. 1942;2:237–239. doi: 10.1136/bmj.2.4260.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Seltzer Z, Dubner R, Shir Y. A novel behavioral model of neuropathic pain disorders produced in rats by partial sciatic nerve injury. Pain. 1990;43:205–218. doi: 10.1016/0304-3959(90)91074-S. [DOI] [PubMed] [Google Scholar]
- 62.Shi TJ, Cui JG, Meyerson BA, Linderoth B, Hokfelt T. Regulation of galanin and neuropeptide Y in dorsal root ganglia and dorsal horn in rat mononeuropathic models: possible relation to tactile hypersensitivity. Neuroscience. 1999;93:741–757. doi: 10.1016/s0306-4522(99)00105-0. [DOI] [PubMed] [Google Scholar]
- 63.Shi TJ, Tandrup T, Bergman E, Xu ZQ, Ulfhake B, Hokfelt T. Effect of peripheral nerve injury on dorsal root ganglion neurons in the C57 BL/6J mouse: marked changes both in cell numbers and neuropeptide expression. Neuroscience. 2001;105:249–263. doi: 10.1016/s0306-4522(01)00148-8. [DOI] [PubMed] [Google Scholar]
- 64.Stone LS, Vulchanova L, Riedl MS, Wang J, Williams FG, Wilcox GL, Elde R. Effects of peripheral nerve injury on alpha-2A and alpha-2C adrenergic receptor immunoreactivity in the rat spinal cord. Neuroscience. 1999;93:1399–1407. doi: 10.1016/s0306-4522(99)00209-2. [DOI] [PubMed] [Google Scholar]
- 65.Sun JH, Yang B, Donnelly DF, Ma C, LaMotte RH. MCP-1 enhances excitability of nociceptive neurons in chronically compressed dorsal root ganglia. J Neurophysiol. 2006;96:2189–2199. doi: 10.1152/jn.00222.2006. [DOI] [PubMed] [Google Scholar]
- 66.Tanaka T, Minami M, Nakagawa T, Satoh M. Enhanced production of monocyte chemoattractant protein-1 in the dorsal root ganglia in a rat model of neuropathic pain: possible involvement in the development of neuropathic pain. Neurosci Res. 2004;48:463–469. doi: 10.1016/j.neures.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 67.Terenghi G. Peripheral nerve regeneration and neurotrophic factors. J Anat. 1999;194:1–14. doi: 10.1046/j.1469-7580.1999.19410001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Uçeyler N, Tscharke A, Sommer C. Early cytokine expression in mouse sciatic nerve after chronic constriction nerve injury depends on calpain. Brain Behav Immun. 2007;21:553–560. doi: 10.1016/j.bbi.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 69.Uchida H, Matsushita Y, Ueda H. Epigenetic regulation of BDNF expression in the primary sensory neurons after peripheral nerve injury: implications in the development of neuropathic pain. Neuroscience. 2013;240:147–154. doi: 10.1016/j.neuroscience.2013.02.053. [DOI] [PubMed] [Google Scholar]
- 70.Vega-Avelaira D, Géranton SM, Fitzgerald M. Differential regulation of immune responses and macrophage/neuron interactions in the dorsal root ganglion in young and adult rats following nerve injury. Mol Pain. 2009;5:70. doi: 10.1186/1744-8069-5-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Verkhratsky A, Butt AM. Chichester, UK: Wiley-Blackwell; 2013. Glial Physiology and Pathophysiology. [Google Scholar]
- 72.Vestergaard S, Tandrup T, Jakobsen J. Effect of permanent axotomy on number and volume of dorsal root ganglion cell bodies. J Comp Neurol. 1997;388:307–312. [PubMed] [Google Scholar]
- 73.Vigneswara V, Berry M, Logan A, Ahmed Z. Caspase-2 is upregulated after sciatic nerve transection and its inhibition protects dorsal root ganglion neurons from apoptosis after serum withdrawal. PLoS One. 2013;8:e57861. doi: 10.1371/journal.pone.0057861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vogel C, Stallforth S, Sommer C. Altered pain behavior and regeneration after nerve injury in TNF receptor deficient mice. J Peripher Nerv Syst. 2006;11:294–303. doi: 10.1111/j.1529-8027.2006.00101.x. [DOI] [PubMed] [Google Scholar]
- 75.White FA, Jung H, Miller RJ. Chemokines and the pathophysiology of neuropathic pain. Proc Natl Acad Sci U S A. 2007;104:20151–20158. doi: 10.1073/pnas.0709250104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Whiteside G, Doyle CA, Hunt SP, Munglani R. Differential time course of neuronal and glial apoptosis in neonatal rat dorsal root ganglia after sciatic nerve axotomy. Eur J Neurosci. 1998;10:3400–3408. doi: 10.1046/j.1460-9568.1998.00346.x. [DOI] [PubMed] [Google Scholar]
- 77.Wiberg R, Novikova LN, Kingham PJ. Evaluation of apoptotic pathways in dorsal root ganglion neurons following peripheral nerve injury. Neuroreport. 2018;29:779–785. doi: 10.1097/WNR.0000000000001031. [DOI] [PubMed] [Google Scholar]
- 78.Xiao HS, Huang QH, Zhang FX, Bao L, Lu YJ, Guo C, Yang L, Huang WJ, Fu G, Xu SH, Cheng XP, Yan Q, Zhu ZD, Zhang X, Chen Z, Han ZG, Zhang X. Identification of gene expression profile of dorsal root ganglion in the rat peripheral axotomy model of neuropathic pain. Proc Natl Acad Sci U S A. 2002;99:8360–8365. doi: 10.1073/pnas.122231899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xie J, Ho Lee Y, Wang C, Mo Chung J, Chung K. Differential expression of alpha1-adrenoceptor subtype mRNAs in the dorsal root ganglion after spinal nerve ligation. Brain Res Mol Brain Res. 2001;93:164–172. doi: 10.1016/s0169-328x(01)00201-7. [DOI] [PubMed] [Google Scholar]
- 80.Xu XF, Zhang DD, Liao JC, Xiao L, Wang Q, Qiu W. Galanin and its receptor system promote the repair of injured sciatic nerves in diabetic rats. Neural Regen Res. 2016;11:1517–1526. doi: 10.4103/1673-5374.191228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang X, Chen Y, Wang C, Huang LY. Neuronal somatic ATP release triggers neuron-satellite glial cell communication in dorsal root ganglia. Proc Natl Acad Sci U S A. 2007;104:9864–9869. doi: 10.1073/pnas.0611048104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang X, Dagerlind A, Elde RP, Castel MN, Broberger C, Wiesenfeld-Hallin Z, Hökfelt T. Marked increase in cholecystokinin B receptor messenger RNA levels in rat dorsal root ganglia after peripheral axotomy. Neuroscience. 1993;57:227–233. doi: 10.1016/0306-4522(93)90057-m. [DOI] [PubMed] [Google Scholar]