Figure 1.
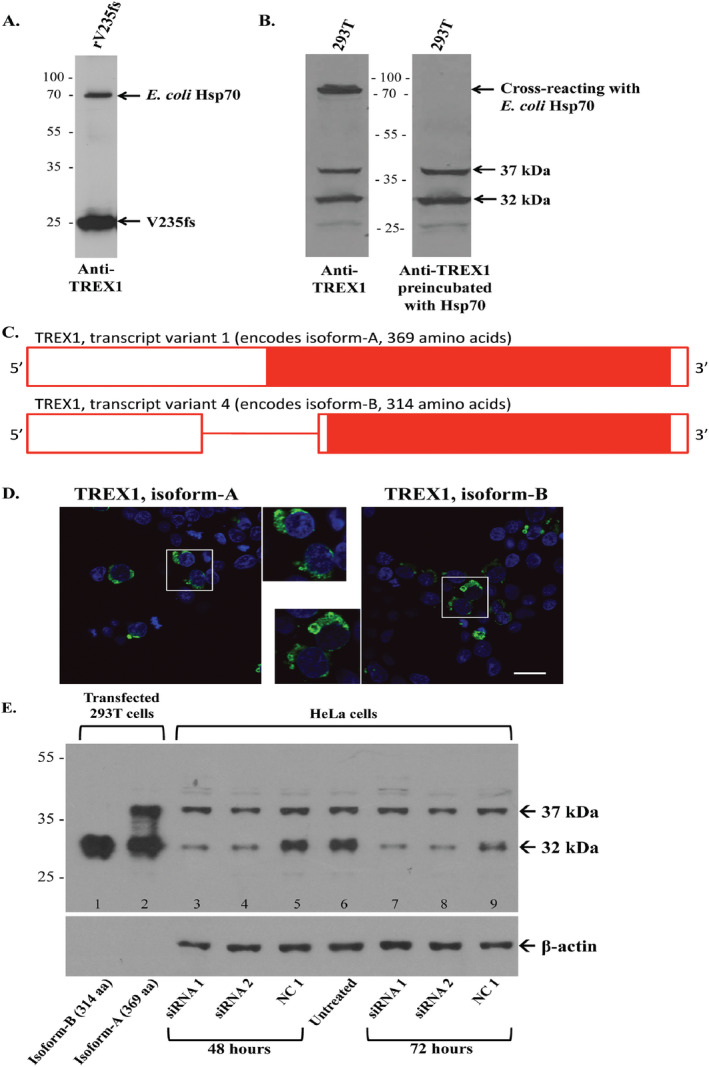
Expression and detection of TREX1 proteins. A. The rabbit anti‐TREX1 Ab detects the recombinant TREX1 (rV235fs) as well as E. coli heat shock protein 70 (Hsp70) in the immunogen on Western blot (WB). B. On strips from the same WB, the anti‐TREX1 Ab detects a band at ~75 kDa in 293T cells (left panel) that is eliminated after pre‐incubation with E. coli Hsp70. The bands at 37 and 32 kDa are unchanged. C. A schematic of TREX1 transcript variants with solid red bar for coding sequence, open red bar for untranslated regions, and red line for intron. Transcript variant 1 retains the intron and encodes a longer N‐terminus. D. 293T cells transiently transfected with fluorescent protein (FP)‐tagged wild‐type TREX1 (green) for isoforms‐A (left panel) and B (right panel). Nuclei are counterstained with TO‐PRO‐3 (blue). Scale bar represents 28 μm. E. Representative WB of TREX1 from 293T cells transfected with wild‐type TREX1 isoforms‐B (lane 1) and A (lane 2) for positive controls and HeLa cells transfected with TREX1 siRNA 1 (lanes 3 and 7) or 2 (lanes 4 and 8), negative control (NC1, lanes 5 and 9) as well as an untreated control (lane 6). Cell lysates were evaluated at 48 (lanes 3–5) and 72 (lanes 7–9) h after transfection with siRNA. Membrane was stripped and reprobed with anti‐β‐actin Ab as a loading control.
