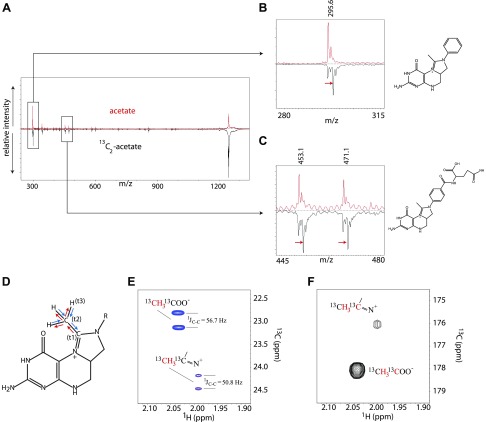
Figure 4.
L. reuteri produces 5,10-methylmethenyl (ethenyl) THF. A–C) MALDI-TOF/TOF MS/MS spectra (A) of SAX purified ethenyl THF Glu6 localizes the acetate-derived methyl group into the folate head group (B, C). D–F) NMR experiments. D) In the 3D HMCMCO experiment, the magnetization is transferred from methyl proton to methyl carbon, and then to the imidine carbon (blue arrows). After chemical shift labeling of the imidine carbon (t1), the magnetization is transferred back to methyl carbon (red arrows) followed by the chemical shift labeling (t2), and then back to methyl proton with proton detection (t3). E) Overlay of the 1H-[13C] HSQC spectra of the methyl groups from acetate with 5,10-ethenyl-THF (in blue) and 5,10-ethenyl-THF alone (in red). The signal splittings are from carbon-carbon 1-bond J couplings. F) Cross peak between methyl proton and carbonyl carbon in acetate, and imidine carbon in 5,10-ethenyl-THF. Red characters in E and F mark the 1H and [13C] nuclei from which the cross peaks were obtained.