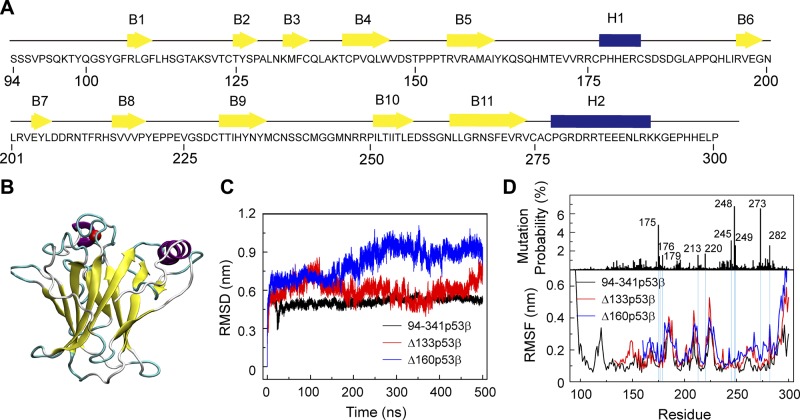
Figure 1.
∆133p53β and Δ160p53β are destabilized because of truncation of core domain residues, whereas the effect on Δ133p53β is much smaller than that on Δ160p53β system. A) The sequence of the wt p53 DBD. β-strands and α-helices are represented by yellow arrows and blue cylinders, respectively. B) The 3D structure of the p53 DBD (residues 94–300). C) Time evolution of Cα RMSD of the p53 core domain (residues 96–300 for 94-341p53β, residues 135–300 for Δ133p53β, and residues 162–300 for Δ160p53β) in the 3 systems. D) The single site residue mutation frequencies of cancer-associated p53 and average Cα RMSF of 94-341p53β, Δ133p53β and Δ160p53β.