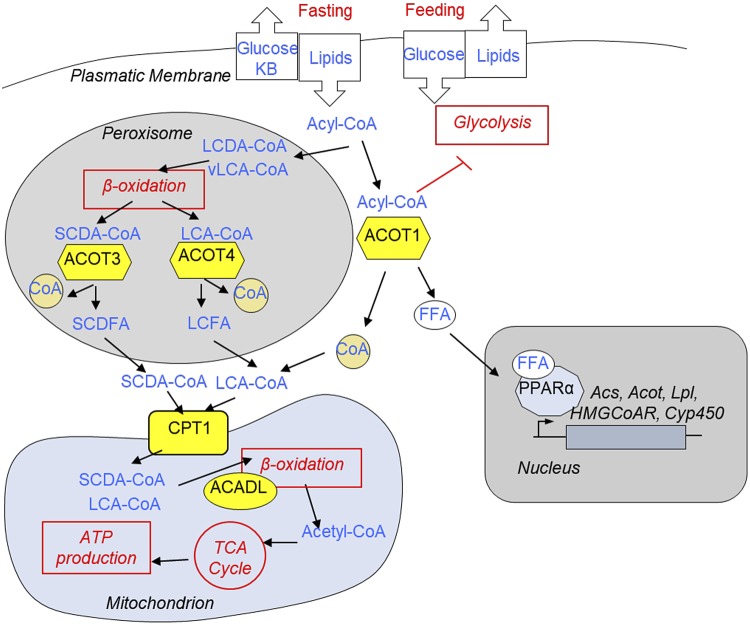
Figure 8.
Reprogramming of fatty acid metabolism by CR (hypothesis). CR is a state of periodic feeding and dietary restriction. During dietary restriction, there is significant influx of fatty acids in the liver. Activated fatty acids—Acyl-CoA—can be used to produce ketone bodies or oxidized in mitochondria to generate ATP for hepatocyte homeostasis and to fuel gluconeogenesis. vLCA-CoA and LCDA-CoA molecules are oxidized in the peroxisomes. It was proposed that after few rounds of carbon chain shortening of vLCA-CoA and LCDA-CoA, ACOTs would localize in peroxisome (i.e., ACOT3 and ACOT4) hydrolyze LCA-CoA and SCDFA-CoA. Generated short-chain and long-chain FFA molecules leave the peroxisome, get activated in the cytoplasm and through carnitine dependent shuttle (catalyzed by CPT1), and enter into the mitochondria. In mitochondria, Acyl-CoA molecules undergo β-oxidation (ACADL catalyzes the rate-limiting step), and generated Acyl-CoA enters the TCA cycle to produce ATPs. Cytoplasmic ACOT1 hydrolyzes Acyl-CoA into FFAs and CoA. It was proposed that this can serve several purposes: limit the supply of Acyl-CoA to mitochondria and reduce the level of cytoplasmic acyl-CoA molecules known to inhibit glycolysis, thus contributing to metabolic flexibility. Finally, it will provide FFAs that serve as ligands for nuclear receptor PPARα, master regulator of fat metabolism. Only the enzymes whose translation has been affected by CR in our study (yellow) are presented in the figure.