Abstract
Pichia pastoris (Komagataella sp.) is broadly used for the production of secreted recombinant proteins. Due to the high rate of protein production, incorrectly folded proteins may accumulate in the endoplasmic reticulum (ER). To restore their proper folding, the cell triggers the unfolded protein response (UPR); however, if the proteins cannot be repaired, they are degraded, which impairs process productivity. Moreover, a non-producing/non-secreting subpopulation of cells might occur, which also decreases overall productivity. Therefore, an in depth understanding of intracellular protein fluxes and population heterogeneity is needed to improve productivity. Under industrially relevant cultivation conditions in bioreactors, we cultured P. pastoris strains producing three different recombinant proteins: penicillin G acylase from Escherichia coli (EcPGA), lipase B from Candida antarctica (CaLB) and xylanase A from Thermomyces lanuginosus (TlXynA). Extracellular and intracellular product concentrations were determined, along with flow cytometry-based single-cell measurements of cell viability and the up-regulation of UPR. The cell population was distributed into four clusters, two of which were viable cells with no UPR up-regulation, differing in cell size and complexity. The other two clusters were cells with impaired viability, and cells with up-regulated UPR. Over the time course of cultivation, the distribution of the population into these four clusters changed. After 30 h of production, 60% of the cells producing EcPGA, which accumulated in the cells (50–70% of the product), had up-regulated UPR, but only 13% of the cells had impaired viability. A higher proportion of cells with decreased viability was observed in strains producing CaLB (20%) and TlXynA (27%). The proportion of cells with up-regulated UPR in CaLB-producing (35%) and TlXynA-producing (30%) strains was lower in comparison to the EcPGA-producing strain, and a smaller proportion of CaLB and TlXynA (<10%) accumulated in the cells. These data provide an insight into the development of heterogeneity in a recombinant P. pastoris population during a biotechnological process. A deeper understanding of the relationship between protein production/secretion and the regulation of the UPR might be utilized in bioprocess control and optimization with respect to secretion and population heterogeneity.
Keywords: unfolded protein response (UPR), stress response, Pichia pastoris, super folder green fluorescent protein (sfGFP), flow cytometry, single-cell, fed-batch culture, heterogeneity
Introduction
The yeast Pichia pastoris (Komagataella phaffii) is an established host for the biotechnological production of wide range of heterologous proteins, most of which are secreted (Cereghino and Cregg, 2000; Ahmad et al., 2014; Meehl and Stadheim, 2014; Juturu and Wu, 2018). The knowledge of this host and its use for the production of heterologous proteins is already advanced, but a more systematic and complex understanding of the P. pastoris cell factory is still needed, especially of intracellular metabolite fluxes, regulatory pathways and secretory machinery (Puxbaum et al., 2015; Zahrl et al., 2017). High-level expression of a heterologous gene may lead to an overload of the protein folding capacity of the endoplasmic reticulum (ER), and consequently to an accumulation of unfolded and/or misfolded proteins in the ER. As a response aiming to restore proper protein folding in the ER, and thus to eliminate the ER stress, the unfolded protein response (UPR) is triggered (Mattanovich et al., 2004; Guerfal et al., 2010). When the unfolded proteins cannot be repaired, they are eliminated within the ER-associated protein degradation (ERAD) pathway (Zahrl et al., 2018). The proportion of intracellularly degraded protein may be massive, up to 60% of the total (Pfeffer et al., 2011). Also, an interplay between protein synthesis and degradation to control protein homeostasis remains unclear, but was recently investigated in mammalian cells at single-cell level (Alber et al., 2018). The rate of protein degradation was shown to vary between cells (Alber et al., 2018). In a recombinant strain of P. pastoris, the co-existence of more sub-populations in terms of production and secretion of the recombinant protein was observed, where only part of the population actively secreted (Love et al., 2012). There was a sub-population with a stochastically changing rate of secretion (Love et al., 2010), a sub-population producing different amounts of the recombinant protein (Broger et al., 2011), and one not producing any at all (Broger et al., 2011). Intracellular protein accumulation and/or degradation, together with the existence of a non-secreting phenotype results in decreased productivity of a heterologous protein. Though an important aspect of productivity and approach to a strain's optimization, little attention is typically paid to intracellular product fluxes, stress responses (UPR, ERAD), strain heterogeneity in terms of growth and production/secretion, and cell physiology in microbial cultivation processes under industrially relevant cultivation conditions (Theron et al., 2018).
The UPR was investigated in P. pastoris at the point of clone selection (Aw et al., 2017), and in strains producing different recombinant proteins during fed-batch (Hohenblum et al., 2004; Resina et al., 2007; Sjöblom et al., 2012; Vogl et al., 2014; Zhong et al., 2014; Wang et al., 2017; Yu et al., 2017) or chemostat (Gasser et al., 2007; Hesketh et al., 2013; Rebnegger et al., 2014) bioreactor cultivations. Several recombinant proteins were shown to up-regulate UPR in P. pastoris, e.g., antibody fragments (Gasser et al., 2007; Khatri et al., 2011), human lysozyme (Hesketh et al., 2013), human trypsinogen (Hohenblum et al., 2004), lipase from Rhizopus oryzae (Resina et al., 2007), mucin-type protein fused with green fluorescent protein (GFP) (Sjöblom et al., 2012), membrane transporter proteins (Vogl et al., 2014), prolyl endopeptidase (Wang et al., 2017), phospholipase A2 from Streptomyces violaceoruber (Yu et al., 2017) or human interleukin (Zhong et al., 2014). In contrast, the production of human serum albumin did not lead to induction of UPR (Hohenblum et al., 2004; Aw et al., 2017).
In P. pastoris, the UPR has so far been studied at the level of transcript of the genes involved in the UPR, using RNAseq (Yu et al., 2017), qPCR (Guerfal et al., 2010; Khatri et al., 2011; Sjöblom et al., 2012; Zhong et al., 2014; Wang et al., 2017), TRAC (Gasser et al., 2007), microarrays (Graf et al., 2008; Dragosits et al., 2010; Rebnegger et al., 2014; Vogl et al., 2014; Edwards-Jones et al., 2015; Aw et al., 2017) or sandwich hybridization assays (Resina et al., 2007). Alternatively, the UPR was detected at the level of protein with 2D electrophoresis (Dragosits et al., 2010; Vanz et al., 2014), Western blot (Zhong et al., 2014) or flow cytometry after immunofluorescent staining (Hohenblum et al., 2004). These methods can be laborious, time-consuming and expensive. Additionally, detection of the intracellular content of mRNA or protein requires previous cell disruption, which hinders the UPR monitoring during a running bioprocess. Recently, indirect detection of UPR levels based on metabolite biomarkers was suggested. This method can be applied in a high-throughput manner, and thus is more suitable for the screening scale (Tredwell et al., 2017). In yeast cell factories other than P. pastoris, easily detectable reporter proteins like β-galactosidase (Cox et al., 1993; Madzak and Beckerich, 2006) or super folder green fluorescent protein (sfGFP) (Lajoie et al., 2012) were previously used to monitor the UPR.
During fed-batch bioreactor cultivations of recombinant P. pastoris strains producing penicillin G acylase from Escherichia coli (EcPGA), lipase B from Candida antarctica (CaLB) or xylanase A from Thermomyces lanuginosus (TlXynA), we systematically analyzed the production (secretion vs. intracellular accumulation) of the recombinant protein. Moreover, with flow cytometry, i.e., at the single-cell level, at-line and in a non-invasive manner, we also analyzed the up-regulation of UPR, cell physiology and heterogeneity of the P. pastoris strains. To monitor the up-regulation of UPR in the P. pastoris strains, a plasmid bearing a gene for sfGFP under the control of the KAR2 promoter was integrated into the P. pastoris genome. The sfGFP is a fast and robustly folding variant of GFP that is synthesized within a few minutes (Pédelacq et al., 2006; Khmelinskii et al., 2012), which makes it an appropriate biosensor for the immediate detection of folding events in the cell. KAR2 is a gene involved in UPR, and its product, Kar2p protein, is an ER-resident chaperone that recognizes misfolded/unfolded proteins in the ER and assists proper protein folding (Dudek et al., 2009). Using flow cytometry for the detection of the sfGFP fluorescent signal, it was possible to monitor the activation of the KAR2 promoter, i.e., up-regulation of the UPR at-line during the cultivation process.
Materials and Methods
Culture Media
YPD medium contained 20 g glucose, 20 g peptone, 10 g yeast extract and 15 g agar per liter. YPD medium with 0.1 mg mL−1 Zeocin™ (Invitrogen, Carlsbad, USA) was used for the selection of the transformants containing the pPICZ-α-A plasmid with different recombinant genes. YPD medium with 0.1 mg mL−1 Nourseothricin (Jena Bioscience, Jena, Germany) was used for the selection of the strains containing the pREP-UPSKAR2-sfGFP-NAT plasmid.
BMG (buffered minimal medium with glycerol) was used for screening the P. pastoris clones with integrated pREP-X-sfGFP-NAT or pREP-UPSKAR2-sfGFP-NAT plasmid and for the flask cultivation of the P. pastoris strain producing EcPGA (section 3.3). BMG medium contained 10 g glycerol, 13.4 g yeast nitrogen base with ammonium sulfate and without amino acids (YNB), and 0.4 mg biotin per liter and 100 mM potassium phosphate buffer (pH 6.0).
BMD1% (buffered minimal medium with 1% dextrose), BMM10 and BMM2 (buffered minimal media with 5 and 1% methanol, respectively) were used for the micro-scale cultivation screening in 96-deep well plates (Weis et al., 2004). The BMD1% medium contained 10 g glucose, 13.4 g YNB and 0.4 mg biotin per liter and 200 mM potassium phosphate buffer (pH 6.0). The BMM10 medium contained 50 mL methanol, 13.4 g YNB and 0.4 mg biotin per liter and 200 mM potassium phosphate buffer (pH 6.0). The BMM2 medium contained 10 mL methanol, 13.4 g YNB and 0.4 mg biotin per liter and 200 mM potassium phosphate buffer (pH 6.0).
BMGY (buffered complex medium with glycerol) was used for growth of the inoculum for the bioreactor cultivations. BMGY medium contained 10 g glycerol, 10 g yeast extract, 20 g peptone, 13.4 g YNB and 0.4 mg biotin per liter and 100 mM potassium phosphate buffer (pH 6.0).
Defined mineral medium used for batch bioreactor cultivations contained 30 g glycerol as a sole carbon source, 2.86 g K2SO4, 0.64 g KOH, 2.32 g MgSO4·7H2O, 0.17 g CaSO4·2H2O, 0.60 g EDTA disodium dehydrate, 7.23 g 85% H3PO4, 0.22 g NaCl, 0.1 mL polypropylene glycol (PPG), 4.35 mL filter sterilized PTM1 solution and 4.35 mL filter sterilized biotin solution per liter. The PTM1 stock solution contained 5.0 mL 96% H2SO4, 3.84 g CuSO4, 0.08 g NaI, 3.0 g MnSO4·H2O, 0.2 g Na2MoO4·2H2O, 0.02 g H3BO3, 0.92 g CoCl2·6H2O, 20.0 g ZnCl2, and 65.0 g FeSO4·7H2O per liter. The biotin stock solution contained 0.20 g L−1 biotin. Before inoculation, the pH of the medium was adjusted to 5.5 using 8.5% NH4OH.
Concentrated medium used in the first phase of fed-batch (growth phase) contained 588 g glycerol, 12 mL of filter sterilized PTM1 solution and 12 mL of filter sterilized biotin solution per 1,000 g feed solution. Concentrated medium used in the second phase of fed-batch (production phase) contained 1,000 g methanol, 12 mL of filter sterilized PTM1 solution and 12 mL of filter sterilized biotin solution per 1,000 g feed solution.
Plasmids
Construction of the UPR-Reporter Plasmid pREP-UPSKAR2-sfGFP-NAT
The plasmid for monitoring the UPR in recombinant P. pastoris strains, named pREP-UPSKAR2-sfGFP-NAT, carried a 324 base pair (bp) upstream region of the KAR2 coding sequence containing one copy of the unfolded protein responsive element (UPRE) sequence, the sfGFP coding sequence (Khmelinskii et al., 2012), the nourseothricin acetyl transferase gene (nat) and the HIS4 gene for integration of the plasmid into the P. pastoris HIS4 locus. The construction of this plasmid is described in detail in Supplementary Figure 1. The plasmid map is provided in Supplementary Files.
Construction of Plasmids Bearing the Genes of the Model Recombinant Proteins
The expression cassettes for recombinant protein production contained the AOX1 promoter, a secretion signal, the coding sequence of the heterologous gene (EcPGA, CaLB, TlXynA), the AOX1 terminator and the Zeocin resistance cassette. In the case of EcPGA and CaLB, the α-factor secretion signal from S. cerevisiae was used as the secretion signal, and in the case of TlXynA, the natural secretion signal from Thermomyces lanuginosus was kept (Mellitzer et al., 2012b).
Construction of the expression plasmid carrying the EcPGA gene (Sobotková et al., 1996) (pPICZαA_EcPGA) is described elsewhere (Borcinova et al., in preparation). Briefly, the coding sequence of the EcPGA gene was codon optimized for P. pastoris and cloned into the pPICZα A plasmid (Invitrogen, Carlsbad, USA) via XhoI and XbaI restriction sites. The coding sequence of the CaLB gene, codon optimized for P. pastoris, was PCR-amplified from the plasmid pBSY3S1Z_CaLB-WT (bisy e.U., Hofstätten an der Raab, Austria) using primers alphaMF-CALB_fw and CALB-AOX1TT_rev, and cloned into pBSYAOXsec_blunt containing the α-factor secretion signal from S. cerevisiae (bisy e.U., Hofstätten an der Raab, Austria) via MlyI restriction sites. The constructed plasmid was named pBSYAOXsec_CaLB. The natural secretion signal and the coding sequence of TlXynA, codon optimized for P. pastoris, were PCR-amplified from the gBlock XylA_opt using primers PAOX1-XYL_fw and XYL-AOX1TT_rev, and cloned into pBSYAOX_blunt (bisy e.U., Hofstätten an der Raab, Austria) via MlyI restriction sites. The constructed plasmid was named pBSYAOX_TlXynA.
The nucleotide sequences of all the above-mentioned primers are provided in Supplementary Table 1. The plasmid maps are provided in Supplementary Files.
Strains
Electro-competent P. pastoris X33 (Invitrogen) cells were prepared and transformed [according to the protocol described by Lin-Cereghino et al. (2005)] with the plasmid DNA and the cells were regenerated in 1 mL of a 1:2 mixture of 1 M sorbitol and YPD medium for 2 h. For the transformation of P. pastoris cells with the UPR-reporter or -control plasmid, ~500 ng of SalI-linearized plasmid pREP-UPSKAR2-sfGFP-NAT or pREP-X-sfGFP-NAT was used. The transformants were plated out on selective YPD-agar plates with 0.1 mg mL−1 nourseothricin (Jena Bioscience, Jena, Germany) and incubated for 2–3 days at 28°C. Selected transformants were cultured overnight in 2 mL BMG at 250 rpm and 28°C, and the production of sfGFP was analyzed by flow cytometry (see chapter Flow Cytometry). An average-expressing clone from each construct was used for further studies. For the transformation of P. pastoris cells with the plasmid carrying the EcPGA gene, pPICZαA_EcPGA, ~5 μg of the linearized plasmid DNA were used and the transformants were selected on YPD-agar plates with Zeocin (Invitrogen, Carlsbad, USA) (Borcinova et al., in preparation). For the transformation of P. pastoris cells with the plasmids carrying the CaLB or TlXynA genes, ~2 μg of PmeI-linearized plasmid pBSYAOXsec_CaLB or pBSYAOX_TlXynA, respectively were used for the transformation. The transformants were plated out on selective YPD-agar plates with 0.1 mg mL−1 Zeocin (Invitrogen, Carlsbad, USA) and incubated for 2–3 days at 28°C. Random transformants were screened in 96-deep well plates (see chapter Micro-Scale Cultivation Screening). All strains used in this study were stored in 24% glycerol at −80°C.
Micro-Scale Cultivation Screening
P. pastoris clones producing CaLB or TlXynA were cultured in 96-deep well plates in shakers (INFORS Multitron, Bottmingen, Switzerland) at 28°C, 320 rpm, and 80% relative humidity (Weis et al., 2004). After an initial batch phase for 60 h in 250 μL BMD1%, the cultures were induced with addition of 250 μL BMM2, i.e., 0.5% methanol. After 12, 24, and 48 h from the first induction, 50 μL of BMM10 was added to keep the methanol concentration at app. 0.5%. After 60 h from the first induction with methanol, the culture was centrifuged (4,000 rpm, 15 min, 4°C) and the enzymatic activity of CaLB or TlXynA was determined in the supernatant according to the protocols described in chapter Protein Analysis. From each construct, the clone with the highest enzymatic activity, irrespective of the number of integrated gene copies (not determined), was used for further studies.
Bioreactor Cultivations
Batch Cultivations for Determination of Growth Characteristics
A glycerol stock (1 mL) of the strain (all listed in Table 1) was thawed and used to inoculate 100 mL of BMGY medium. This first seed culture was grown for 48 h at 30°C and 150 rpm, and then 20 mL of the culture were used to inoculate 180 mL of sterile BMGY (i.e., 10% inoculation ratio). This second seed culture was grown for 12 h at 30°C and 150 rpm, and then aseptically transferred to a 3.6-liter bioreactor (Infors AG, Bottmingen, Switzerland) to achieve a 10% inoculation ratio. The batch volume was 2 L. After glycerol (30 g per liter) was consumed, i.e., after the end of glycerol batch, a batch phase with methanol was performed, which was initiated by pulsing 15 g of methanol per liter to the culture. The maximum specific growth rate of biomass and biomass/substrate yield with glycerol and methanol were calculated from the data obtained from the batch cultivation with glycerol and methanol, respectively (see chapter Data Analysis) for each strain, and were comparable for all strains (Supplementary Table 2).
Table 1.
Overview of the fed-batch cultivations.
| Recombinant protein | None | EcPGA | CaLB | TlXynA | ||||
|---|---|---|---|---|---|---|---|---|
| Strain | Pp1 (control) | Pp1 (control) | Pp4 | Pp5 (control) | Pp10 | Pp10 | Pp10 | Pp14 |
| sfGFP expression | PKAR2 | PKAR2 | PKAR2 | No promoter (x) | PKAR2 | PKAR2 | PKAR2 | PKAR2 |
| μmethanol (h−1) set | 0.016 | 0.032 | 0.016 | 0.016 | 0.016 | 0.032 | 0.008 | 0.016 |
| μmethanol (h−1) reached | 0.021 ± 0.001 | 0.039 ± 0.001 | 0.019 ± 0.002 | 0.021 ± 0.002 | 0.018 ± 0.001 | 0.036 ± 0.001 | 0.007 ± 0.001 | 0.019 ± 0.001 |
P. pastoris strains producing three different recombinant proteins and/or containing a reporter of UPR up-regulation were constructed. The recombinant proteins were EcPGA, CaLB, and TlXynA. The reporter of the up-regulation of UPR was a genome-integrated cassette consisting of the KAR2 promoter and sfGFP gene. Three strains contained the UPR reporter and produced a recombinant protein—EcPGA (Pp4), CaLB (Pp10), or TlXynA (Pp14). A control strain to verify the suitability of our UPR reporter produced EcPGA and contained a genome-integrated cassette carrying the sfGFP gene, but no promoter in front of this gene (Pp5). A control strain to examine the UPR up-regulation by cultivation conditions contained the UPR reporter, but produced none of the three recombinant proteins of interest (Pp1). In all processes, temperature was maintained at 30°C and pH at 5.5. All processes consisted of a batch phase with glycerol (30 g l−1), a short (2.5 h) growth fed-batch with an exponential feeding of glycerol to maintain the specific growth rate at 0.2 h−1, and a production fed-batch with an exponential feeding of methanol to maintain the specific growth rate at 0.008, 0.016, or 0.032 h−1.
Fed-Batch Cultivations
A glycerol stock (1 mL) of the strain (all listed in Table 1) was thawed and used to inoculate 100 mL of BMGY medium. This first seed culture was grown for 48 h at 30°C and 150 rpm, and then 60 mL of the culture were used to inoculate 540 mL of sterile BMGY (i.e., 10% inoculation ratio). This second seed culture was grown for 12 h at 30°C and 150 rpm, and then aseptically transferred to an 18-liter bioreactor (Bilfinger Industrietechnik, Salzburg, Austria) to achieve a 10% inoculation ratio. All cultivations were carried out as single experiments (i.e., without replication) at 30°C, pH 5.5, 0.5 bar overpressure, 3 VVM airflow (without oxygen enrichment) and agitation speed 1,100 rpm. Ammonium hydroxide (8.5%) and phosphoric acid (8.5%) were used for pH adjustment. The batch volume was 6 L and the end of batch was indicated by a rapid increase in dissolved oxygen concentration in the medium (after ~12 h). To increase the biomass concentration, exponential feeding of a concentrated glycerol solution (58.8% w/w glycerol) was immediately initiated in order to keep specific growth rate μ at a constant value of 0.180 h−1 (app. 80% μmax with glycerol) according to the feeding equation F = 61.88·e0.18·t. After 2.5 h of glycerol feeding, exponential feeding of a concentrated methanol solution (100% w/w methanol) was initiated to keep the specific growth rate μ at a constant value of 0.008 h−1 (app. 15% μmax with methanol), 0.016 h−1 (app. 30% μmax with methanol) or 0.032 h−1 (app. 60% μmax with methanol). The respective feeding equations were F = 7.56·e0.008·t, F = 12.52·e0.016·t and F = 22.44·e0.032·t. The feeding rates were calculated according to equation (3) in chapter Data Analysis. As parameters μmax and Yx/s,max in equation (3), the average value of the maximum specific growth rates and maximum biomass/substrate yields of the strains Pp4 and Pp5 was used (since these strains were cultured as the first ones in the fed-batch cultivations). Samples were withdrawn regularly during fed-batch culture.
Substrate Analyses
The concentrations of glycerol and methanol in the centrifuged cultivation medium (14,000 rpm, 5 min) were determined by high-pressure liquid chromatography (HPLC) using an LC-20AB device equipped with autosampler SIL-20A, thermostated column oven CTO-20A and refractometer detector RID-10A (all produced by Shimadzu). The Aminex HPX-87H column, with an internal diameter (i.d.) of 7.8 mm (Bio-Rad, Munich, Germany), was run at 40°C at a flow rate of 0.6 mL min−1 under isocratic conditions, with 2.5 mM H2SO4 and an injection volume of 25 μL.
Biomass Concentration
The cells were harvested by centrifugation (14,000 rpm, 5 min), washed with distilled water and dried at 105°C until constant weight. The biomass concentration was then determined as cell dry weight (CDW).
Flow Cytometry
A BD Accuri™ C6 flow cytometer (BD Biosciences, Franklin Lakes, USA) equipped with a 20 mW 488 nm solid state blue laser was employed for measurements of the green fluorescence of sfGFP (FL1 530 ± 15 nm) during the flask experiments for the characterization of the KAR2 upstream region (chapter Development of the UPR-Reporter Based on Characterization of the KAR2 Upstream Region) and the for the characterization of the population producing recombinant EcPGA (chapter Evaluation of the Flow Cytometry Data From Bioreactor Cultivations). Prior to analysis, cells were centrifuged (2,000 g, 5 min) and resuspended in phosphate-buffered saline (PBS) to an OD600 of 0.5. The data were analyzed using BD Accuri CFlow® Plus software (BD Biosciences, Franklin Lakes, USA).
Cells from the bioreactor experiments (chapters Evaluation of the Flow Cytometry Data From Bioreactor Cultivations and Physiology- and UPR-Related Effects of Production of Different Heterologous Proteins) were stained with propidium iodide (PI, Invitrogen, 1.0 mg/mL solution in water, P3566) and analyzed using a BD FACSCalibur 4CA flow cytometer (Becton Dickinson GmbH, Heidelberg, Germany) equipped with a 488-nm argon-ion laser, a 635-nm red diode laser, and the appropriate FACSFlow sheath fluid. Propidium iodide was used to assess cell viability as integrity of the cytoplasmic membrane, since PI effectively only enters cells with non-intact or damaged membranes and intercalates into double-stranded nucleic acids, resulting in a red fluorescence; such cells were considered as non-viable. The fluorescence emitted was collected in two optical channels, FL1 (515–545 nm) for the green fluorescence of sfGFP and FL3 (>650 nm) for the red fluorescence of propidium iodide. Samples were taken directly from the culture and were diluted with phosphate-buffered saline (PBS) to reach < 100,000 events per 30 s analysis at a low flow speed (12 μL min−1). The diluted P. pastoris cell suspension was incubated in the BD measuring tube with PI protected from light, and was thus measured directly. BD CellQuest Pro software was used in the cytometer, while the data were analyzed using FlowJo software (Tree Star Inc.).
The flow cytometry data from the bioreactor cultivations (chapters Evaluation of the Flow Cytometry Data From Bioreactor Cultivations, Physiology- and UPR-Related Effects of Production of Different Heterologous Proteins, and The Influence of Specific Growth Rate on CaLB Production, Physiology and ER-Stress) were evaluated in RStudio, using principle component analysis (PCA) in order to reduce redundancy (e.g., cell size-based effects on sfGFP content). With PCA, the natural logarithm (ln) values of the measured flow cytometric data (FSC, SSC, FL1, FL2, FL3) were de-correlated and displayed in a two dimensional system of two principle components (PCs). The combination PC3-PC2, which described about 39.8% of the population variability, showed the lowest level of redundancy and was used for a clustering analysis. The clustering algorithm was developed for a sample of 10,000 measured cells from three different processes (cultivations of the strains producing EcPGA and CaLB, and the non-producing strain with μmethanol 0.016 h−1), using k-means. The number of clusters was allowed to vary from one to six in order to maximize the resulting Dunn index. The remaining measurements were then clustered according to the determined population centers, using a nearest neighbors approach.
Fluorescent Microscopy
For microscopic examination of cell size, morphology and fluorescence, phase contrast and epi-fluorescence microscopy were employed using an Olympus BX51 microscope (Olympus, Tokyo, Japan) equipped with a 120 W mercury vapor arc lamp and a U-MWB2 filter cube (excitation 450–480 nm, emission >515 nm). The microphotographs in Supplementary Figure 6 were shot directly by camera (EOS 600D, Cannon, Tokyo, Japan).
Protein Analysis
Determination of Total Protein Concentration
Quick Start™ Bradford 1x Dye Reagent (Bio-Rad, Hercules, USA) was used for the determination of the total protein concentration (mg L−1). The assay was performed according to the producer's instructions. Briefly, 250 μL of the reagent was mixed with 5 μL of the centrifuged supernatant (14,000 rpm, 5 min) in a 96-well plate, the plate was incubated for 5 min at room temperature and then the absorbance at 595 nm was measured. Bovine gamma-globulin (part of the kit) was used as a standard.
Penicillin G Acylase Activity Assay
The enzymatic activity of PGA was determined by measuring the amount of 6-APA generated by hydrolysis of penicillin G (Gao et al., 2006). 50–300 μL of the centrifuged supernatant (14,000 rpm, 5 min) were mixed with 100 mM sodium phosphate buffer (pH 8.0) to reach a total volume of 2 mL. Then, 1 mL of substrate solution (2% (w/v) penicillin G K+ salt in 100 mM sodium phosphate buffer, pH 8.0) was added and the reaction mixture was incubated at 37°C for 8 min. In 2, 4 and 8 min after the addition of the substrate, 0.5 mL of the reaction mixture was withdrawn and the reaction was immediately stopped by the addition of 3 mL stop solution [mixture of 20% (v/v) acetic acid and 50 mM NaOH in ratio 2:1 (v/v)]. Then, 0.5 mL of p-dimethylamidobenzaldehyde solution in methanol (5 g L−1) was added to the stopped reaction. After 15 min incubation of the mixture at room temperature, its absorbance at 415 nm was measured. One activity unit of PGA (U) is defined as the amount of PGA that is needed to produce 1 μmol 6-APA mL−1 min−1.
For measurement of the intracellular EcPGA, centrifuged cells from a 4 mL sample were washed with 0.1 M sodium phosphate buffer (pH 8.0) and stored at −80°C for a minimum of 1 h. The cell pellet was then resuspended to the original volume (4 mL) and diluted with 0.1 M sodium phosphate buffer (pH 8.0), to reach a maximum absorbance OD600 of 50–100. 1 mL of the diluted cell suspension was mixed with 1 mL of glass beads (0.25–0.50 mm) and vortexed for 20 min. The vortexed sample was centrifuged (8,000 rpm, 5 min, 4°C) and the supernatant was used for the PGA activity assay.
Lipase B Activity Assay
The enzymatic activity of Candida antarctica lipase B (CaLB) was determined according to the colorimetric assay using p-nitrophenyl butyrate (pNPB) as a substrate (Krainer et al., 2012). 20 μL of the enzyme sample (culture supernatant) was transferred into a 96-well microtiter plate, and 180 μL of a freshly prepared pNPB working solution (100 μL pNPB stock solution in 10 mL of 300 mM Tris/HCl buffer, pH 8.0; pNPB stock solution was prepared by mixing 42 μL of pNPB (Sigma Aldrich) with 458 μL of DMSO and stored at −20°C) was added. The kinetics of the reaction were measured immediately at 405 nm for 6 min at 25°C. One activity unit (U) of CaLB was defined as the amount of CaLB that was needed to produce 1 μmol p-nitrophenolate mL−1 min−1.
For the measurement of the intracellular CaLB, centrifuged cells from a 0.5 mL sample were washed with PBS and stored at −20°C. The cell pellet was gently resuspended in Y-PER™ Yeast Protein Extraction Reagent (Thermo Scientific, Rockford, USA) in ratio 2.5 μL Y-PER per 1 mg WCW. The suspension was then incubated in the Eppendorf agitator at room temperature for 30 min and 600 rpm. Then, the sample was centrifuged (14,000 rpm, 10 min) and the supernatant was used for the CaLB activity assay.
Xylanase a Activity Assay
The enzymatic activity of Thermomyces lanuginosus (TlXynA) was determined according to the colorimetric para-hydroxybenzoic acid hydrazide (pHBAH) assay, which is used to detect reducing sugars released from polymers (Mellitzer et al., 2012a). For lignocellulosic substrate conversion, 20 μL of the enzyme sample (culture supernatant) or blank (50 mM sodium citrate buffer, pH 4.8) was mixed with 150 μL of the substrate solution (0.5% xylan in 50 mM sodium citrate buffer, pH 4.8). The mixture was incubated for 30 min at 50°C and 300 rpm. For the subsequent reducing-sugar assay, 50 μL of the substrate conversion reaction was mixed with 150 μL of pHBAH working solution (pHBAH stock solution was 5% w/v pHBAH in 0.5% v/v HCl; pHBAH working solution was prepared by mixing the stock solution with 0.5 M NaOH in 1:4 v/v ratio). The mixture was incubated at 99°C for 2 min, and then cooled to 4°C. Then, the absorption at 410 nm was measured. The activity (U L−1) of TlXynA was calculated from a standard curve, which was obtained after performing the assay with a series of standard solutions (0–200 U L−1) of commercial xylanase from Thermomyces lanuginosus (X2753, Sigma Aldrich, St. Louis, USA).
For the measurement of intracellular TlXynA, centrifuged cells from a 0.5 mL sample were washed with PBS and stored at −20°C. The cell pellet was gently resuspended in Y-PER™ Yeast Protein Extraction Reagent (Thermo Scientific, Rockford, USA) in ratio 2.5 μL Y-PER per 1 mg WCW. The suspension was then incubated in the Eppendorf agitator at room temperature for 30 min and 600 rpm. Then, the sample was centrifuged (14,000 rpm, 10 min) and the supernatant was used for the TlXynA activity assay.
Fluorimetric Measurements
Spark 20M multimode microplate reader (Tecan, Männedorf, Switzerland) was used for measuring fluorescence of sfGFP. The mode Fluorescence Top Reading was used, with the excitation wavelength 485 ± 10 nm and emission wavelength 535 ± 10 nm.
qPCR
The sample taken during the bioreactor cultivation was centrifuged (14,000 rpm, 5 min) and the cell pellet was stored at −80°C. Total RNA was isolated from the cell pellets according to the protocol provided by Invitrogen (http://tools.thermofisher.com/content/sfs/manuals/easyselect_man.pdf). The cDNA was prepared using the iScript cDNA Synthesis Kit (Bio-Rad, Hercules, USA). qPCR was performed in QuantStudio™ 5 Real-Time PCR System (ThermoFisher Scientific, Waltham, USA), using the 5 × HOT FIREPol® EvaGreen® qPCR Mix Plus (ROX) (Solis BioDyne, Tartu, Estonia). Relative expression of KAR2 was monitored (primers q34 and q35), using ACT1 as a reference (primers q24 and q25) and no-template as well as no-RT controls. The nucleotide sequences of the primers are provided in Supplementary Table 1. The fold-change of KAR2 expression was evaluated using the ΔΔct method, relating all expression data to the expression of KAR2 in the non-producing control strain in the sample taken 3 h after induction.
Statistical Analysis and Controls
Measurements of biomass concentration were carried out in duplicate. Flow cytometric measurements, measurements of enzyme activity and total protein concentration, and qPCR measurements were carried out in triplicate. The mean values was calculated and the errors were expressed as standard deviations.
Data Analysis
Maximum specific growth rate was calculated according to equation (1):
| (1) |
where x is the biomass concentration (g L−1) and t is the cultivation time (h).
Maximum biomass/substrate yield was calculated according to equation (2):
| (2) |
where s is the substrate concentration (g L−1).
Exponential feeding rate was calculated according to equation (3):
| (3) |
where F0 is the initial feed rate (L h−1), μ is the required specific growth rate (h−1), V0 is the initial working volume (L), x0 is the initial biomass concentration (g L−1) and s0 is the concentration of carbon source in the feed solution (g L−1).
Specific growth rate during fed-batch was calculated according to equation (4):
| (4) |
where V is the working volume (L).
Approximate volume of supernatant was calculated according to equation (5):
| (5) |
where ρbroth is the density of culture broth (kg L−1), Mx, WCW is the mass of wet cells (g L−1) and ρsupernatant is the density of supernatant that is ~1.03 kg L−1 (Potgieter et al., 2010)
The density of culture broth was calculated according to equation (6):
| (6) |
where xWCW is the concentration of biomass wet cell weight (g L−1).
The concentration of wet biomass (g L−1) was calculated according to equation (7). This equation was determined and verified experimentally for the given strain and culture conditions.
| (7) |
Product/biomass yield during fed-batch was calculated according to equation (8):
| (8) |
where cp is the product concentration (U L−1).
Results
We constructed a reporter for monitoring UPR up-regulation in living P. pastoris cells, which was based on the production of sfGFP upon the activation of the P. pastoris KAR2 promoter. Using this reporter, UPR up-regulation was assessed in P. pastoris strains producing different recombinant proteins (EcPGA, CaLB, TlXynA) during bioreactor fed-batch cultivations with methanol. Besides assessing the concentration of the secreted product in the cultivation medium, we also measured the residual/non-secreted product inside the cells. Flow cytometry was used as an at-line, single-cell and non-invasive method for the assessment of the UPR up-regulation (of the fluorescence of sfGFP), cell viability and population heterogeneity.
Development of the UPR-Reporter Based on Characterization of the KAR2 Upstream Region
We aimed to use the KAR2 upstream region for monitoring UPR in P. pastoris during bioreactor cultivations. The KAR2 promoter within the KAR2 upstream region has not yet been characterized in P. pastoris. So far, one copy of a potential UPRE (unfolded protein responsive element) sequence, which is a binding site of the transcription activator Hac1p of UPR-involved genes (Mori et al., 1996, 1998), was revealed in the KAR2 upstream region of P. pastoris (Guerfal et al., 2010). The potential UPRE sequence is CAGCGTG, starting −84 bp upstream to the KAR2 ATG (Figure 1). We prepared mutated variants (truncation and/or single nucleotide mutations) of the P. pastoris KAR2 upstream region to check for the UPR-function of the potential UPRE (Figure 1). These variants were namely: (1) full length KAR2 upstream region starting −324 bp upstream KAR2 CDS (named FL); (2) truncated variant of the KAR2 upstream region starting −190 bp upstream KAR2 CDS, still containing the native UPRE sequence (named −190 bp); (3) truncated variant of the KAR2 upstream region starting −190 bp upstream KAR2 CDS and with two single mutations (84C → A, 78G → A) disturbing the UPRE sequence (named −190 bp mut.); (4) truncated variant of the KAR2 upstream region starting −77 bp upstream KAR2 CDS, thus lacking the UPRE sequence (named −77 bp). These variants were inserted in front of the sfGFP gene in the pREP-PKAR2-sfGFP plasmid, so the variant of the KAR2 upstream region controlled the expression of sfGFP. Plasmid pREP-x-sfGFP, having no promoter in front of the sfGFP gene, was used as a negative control. The pREP-PKAR2-sfGFP plasmids, as well as the pREP-x-sfGFP control plasmid were stably integrated into the HIS4 locus of the P. pastoris X33 strain.
Figure 1.
- full length KAR2 upstream region (FL, −324 bp);
- truncated variant of KAR2 upstream region, still containing native UPRE sequence (−191 bp);
- truncated variant of KAR2 upstream region with two single mutations (84C→A, 78G→A) in the UPRE sequence (−191 bp mut.); truncated variant of KAR2 upstream region lacking the UPRE sequence (−77 bp).
The strains were cultured in shake flasks with 100 mL YPD overnight to OD600 2.0–3.0. The culture was then split into three parallels and incubated for 2 h. One of the parallels was cultured under normal (non-stress) conditions, while the other two parallels were cultured under experimentally-induced stress conditions, i.e., either in presence of 3 mM dithiothreitol (DTT) or under increased temperature (39°C). The activity of the different KAR2 promoter variants was assessed according to the fluorescence of sfGFP with flow cytometry. Under non-stress conditions, the activities of the mutated KAR2 upstream variants were lower than the activity of the full length (FL) variant (Figure 2), namely 1.2-fold in case of the −190 bp variant, 1.8-fold in case of −190 bp mut. variant and 6.7-fold in case of the −77 bp variant. Because the activity of the variant with the mutated potential UPRE sequence (−190 bp mut. variant) was lower than the activity of its non-mutated counterpart (−190 bp variant) and because the activity dramatically decreased when the potential UPRE sequence was missing in the −77 bp variant, we assume that the CAGCGTG might be the consensus UPRE motif in the P. pastoris KAR2 upstream region, required for the functionality of the KAR2 promoter. Against our expectations, up-regulation of the KAR2 upstream region by DTT treatment was insignificant (Figure 2), even in the case of the FL and −190 bp variants. In contrast to DTT, increased temperature of 39°C led to a strong up-regulation of the promoter activity. Compared to the FL variant, which gave the strongest fluorescence signal at the increased temperature, fluorescence of the −190 bp, −190 bp mut., and −77 bp variants were lower (1.1-fold, 1.4-fold, and 10.2-fold, respectively). However, the increase of fluorescence of the heated (39°C) cells, when compared to the non-treated cells, was the most significant in the case of the −190 bp mut. variant (3.1-fold), while for the FL variant it was 2.3-fold, for the −190 bp variant 2.5-fold and for the −77 bp variant 1.5-fold. Because the activity of the variant with the mutated UPRE (−191 bp mut.) increased even more significantly after heat stress than the activity of the non-mutated variant (−191 bp), we assume that the potential UPRE does not function as a heat shock element. The fluorescence of the negative control strain producing no sfGFP (x), i.e., the background fluorescence signal, was considerably lower in comparison to strains with variants of the KAR2 upstream region. Due to the highest sfGFP levels of the FL variant, we decided to use the plasmid bearing the PKAR2(FL)-sfGFP to monitor the UPR in P. pastoris. The FSC-FL1 dot plots are provided in Supplementary Figure 2.
Figure 2.
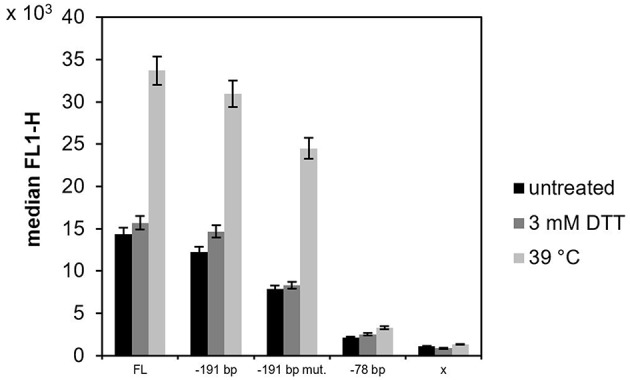
The activity of different variants of the KAR2 upstream region in P. pastoris X33. The promoter activity of the different variants of the KAR2 upstream region was measured as a green fluorescence of sfGFP (channel FL1) with flow cytometry, as the gene of sfGFP was inserted downstream of the KAR2 upstream region. A variant with no promoter in front of the sfGFP gene (x) was used as a negative control. P. pastoris strains with the different variants of the KAR2 upstream region (FL, −190 bp, −190 bp mut., −77 bp) controlling sfGFP expression, and the negative control strain (x-sfGFP) were cultured overnight in shake flasks with YPD, then split into three parallels and further incubated for another 2 h. One parallel was cultured under normal conditions without experimental stress (black bars), the second parallel was cultured in the presence of 3 mM DTT (dark gray bars) and the third parallel was cultured at an increased temperature of 39°C (light gray bars). The displayed values are median FL1 values of all events belonging to a gate defined in a FSC-SSC dot plot (distinguishing P. pastoris cells from the background, data not shown). The error bar is showing a standard deviation of three measurements.
Validation of the PKAR2-Based UPR Reporter in a Strain Over-producing Recombinant Protein in a Bioreactor Fed-Batch Cultivation
The plasmid PKAR2(FL)-sfGFP was integrated into the P. pastoris strain producing EcPGA to monitor the UPR during a bioreactor cultivation. Because the EcPGA was previously observed to intracellularly accumulate in P. pastoris (Borcinova et al., in preparation; Marešová et al., 2017), we assumed that UPR would be up-regulated, and sfGFP would be strongly produced. The up-regulation of UPR upon production of EcPGA was proved both by flow cytometric analysis of sfGFP fluorescence (Supplementary Figures 4, 5) and qPCR analysis of KAR2 expression (Figure 3), when comparing the strain producing EcPGA with a control strain producing no recombinant protein, both having the PKAR2(FL)-sfGFP cassette integrated into their genome.
Figure 3.
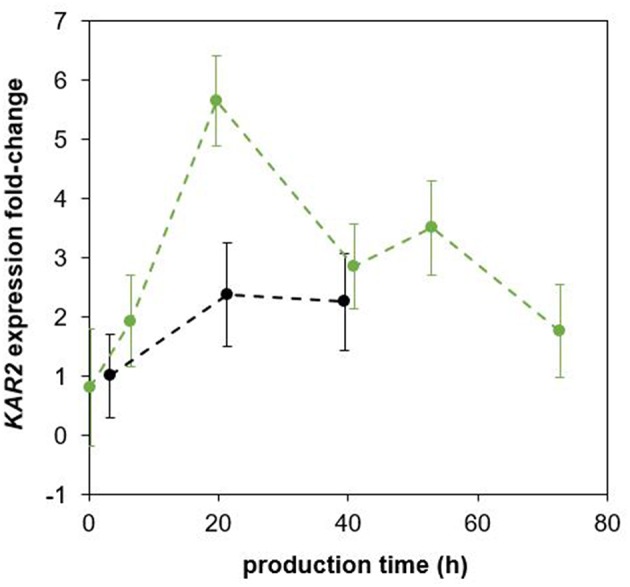
Fold-change of KAR2 expression in the P. pastoris strain producing EcPGA and the non-producing control strain as obtained by qPCR analysis. The P. pastoris strains producing EcPGA (green) and the control non-production strain (black) were cultured under the same cultivation conditions (30°C, pH 5.5), maintaining the specific growth rate of biomass with methanol at 0.016 h−1. All relative expression data are related to the expression of KAR2 in the non-producing control strain in the sample taken 3 h after induction.
Furthermore, we needed to exclude any potential negative effects of sfGFP production on the strain's growth, physiology and production properties, even though it was shown in S. cerevisiae that sfGFP fused to Kar2p did not significantly influence the growth of the strain, nor activated the UPR (Lajoie et al., 2012). Therefore, we prepared a control strain by integrating the negative control cassette x-sfGFP (lacking a promoter upstream the sfGFP, so producing no sfGFP, Figure 2), instead of the UPR reporter cassette (PKAR2(FL)-sfGFP), to the EcPGA-producing strain. The strains were cultured under the same cultivation conditions in bioreactors (Table 1). The specific growth rate with methanol during the production phase (Table 1), the production of EcPGA (Figure 4) and viability (Supplementary Figure 6) were comparable in the two EcPGA-producing strains, suggesting that the production of sfGFP did not impair the growth, production properties, nor viability of the EcPGA-producing strain.
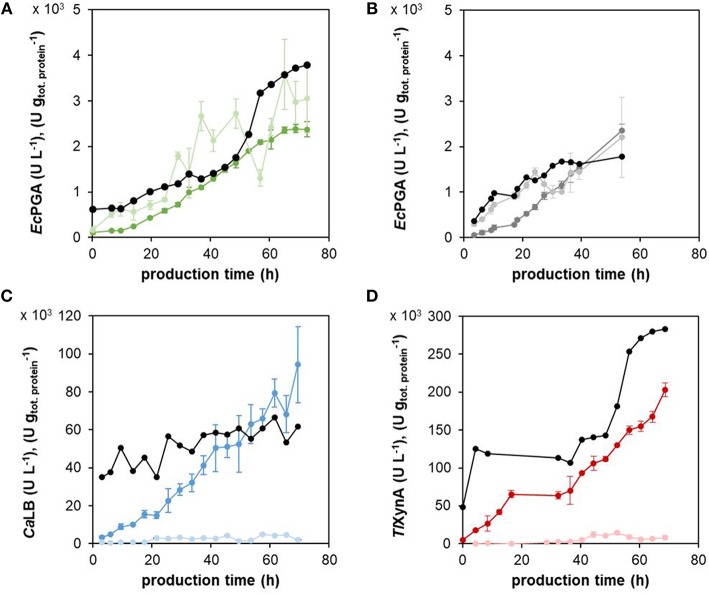
Figure 4.
The enzymatic activity of the different recombinant proteins in the centrifuged culture medium and cell extracts. The production of EcPGA in the strain with the expression cassette PKAR2(FL)-sfGFP (A) and in the control strain (B), CaLB (C), and TlXynA (D) was assessed as enzymatic activity (U L−1) both in the culture medium, i.e., in the supernatant (dark green, dark gray, dark blue, dark red for EcPGA, EcPGA, CaLB, TlXynA, respectively), as well as in the cell extracts, i.e., inside the cells (light green, light gray, light blue, light red for EcPGA, EcPGA, CaLB, TlXynA, respectively). The concentrations were recalculated to the volume of the culture broth. The activities of the extracellular (secreted) enzyme were related to the mass of total extracellular protein (U g, black in A–D). The strains with the integrated PKAR2(FL)-sfGFP cassette for the monitoring of UPR and producing EcPGA (A), CaLB (C), or TlXynA (D), and the control strain with the integrated x-sfGFP cassette producing EcPGA (B) were cultured under the same cultivation conditions (30°C, pH 5.5, μmethanol 0.016 h−1).
Since the production of sfGFP was shown not to influence the strain's physiology and turned out to be detectable with flow cytometry upon the up-regulation of the UPR, we decided to use the PKAR2(FL)-sfGFP reporter system to monitor the UPR in two other recombinant strains, producing CaLB and TlXynA.
Evaluation of the Flow Cytometry Data From Bioreactor Cultivations
The P. pastoris strains producing and secreting EcPGA, CaLB and TlXynA with the integrated PKAR2(FL)-sfGFP cassette, the control strain with the integrated PKAR2(FL)-sfGFP cassette producing none of the recombinant proteins, as well as the control strain without the integrated control x-sfGFP cassette producing EcPGA were cultured under the same cultivation conditions (30°C, pH 5.5) during fed-batch cultivations with methanol, maintaining μmethanol at 0.016 h−1 (ca 30% of μmax, methanol of the strains). The achieved specific growth rate with methanol of all the strains was comparable (Table 1).
Flow cytometry was used to assess cell size and complexity (FSC and SSC, respectively), green fluorescence of sfGFP (FL1) referring to the activity of the KAR2 promoter (UPR), and cell viability as red fluorescence of PI bound within cells with compromised cell membranes (FL3) during the cultivation. Two sub-populations with respect to the FSC signal were distinguished and observed in all strains over the production phase with methanol (examples of density plots are shown in Supplementary Figure 3). It is apparent from the FSC-FL1 dot plot overlay (Supplementary Figure 4) that the FL1 signal (fluorescence of sfGFP) correlated with FSC to some extent, and that the increase in FL1 over time was more significant in the case of cells with higher FSC. The time course of sfGFP fluorescence (median FL1) evaluated separately for the two sub-populations with different sizes (FSC) for all strains is provided in Supplementary Figure 5.
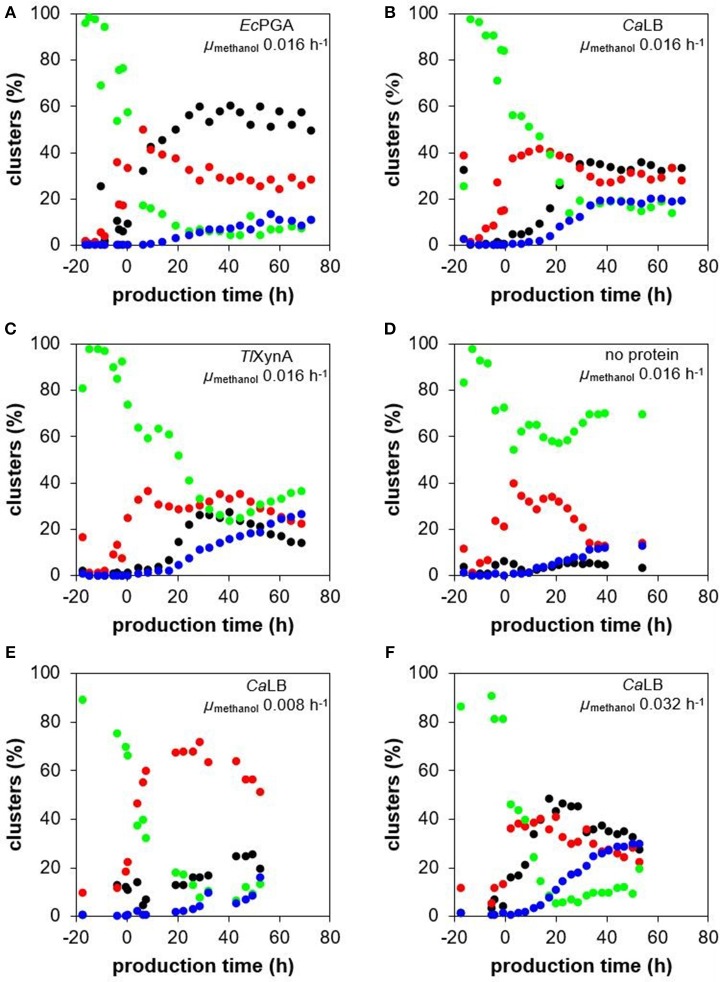
In order to reduce redundancy, such as for example the effect of cell size (FSC) on the sfGFP content (FL1) (see Supplementary Figure 4), the flow cytometric data were further evaluated using PCA (Figure 5). Based on the PCA, the following four sub-populations were distinguished: (I) smaller and less complex (lower FSC and SSC) viable cells with no UPR up-regulation; (II) larger and more complex (higher FSC and SSC, also including budding cells, i.e., two incompletely separated cells) viable cells with no UPR up-regulation; (III) viable cells with up-regulated UPR (increased FL1); and (IV) cells with impaired viability (increased FL3).
Figure 5.
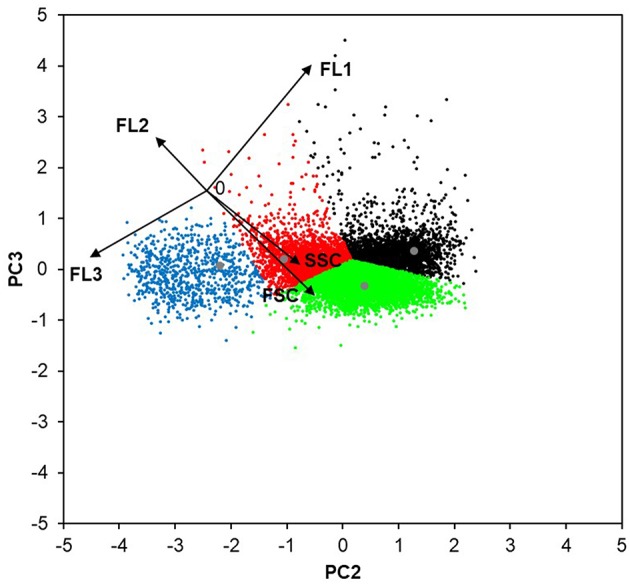
The four sub-populations of P. pastoris cells defined with the use of PCA. The flow cytometric data (FSC, SSC, FL1, FL2, FL3) of 10,000 random cells from the bioreactor cultivations of the strains producing EcPGA and CaLB, and the non-producing strain (all at μmethanol 0.016 h−1) were analyzed with PCA. The lowest level of redundancy was observed when the data were displayed as the combination of PC2 and PC3 (describing ~39.8% of the population variability), since the flow cytometric data (black vectors) were the most de-correlated. The length of each vector is 50 AU. The combination of PC3 and PC2 was used to establish the clustering method (population centers are displayed in gray). According to the FC signals (black arrows), the identified four clusters can be described as follows: smaller and less complex viable cells with no UPR up-regulation (red); larger and more complex viable cells with no UPR up-regulation (green); viable cells with up-regulated UPR (black); and cells with impaired viability (blue).
In order to additionally support the flow cytometry-based results indicating the heterogeneity of P. pastoris population with respect to cell size/complexity (FSC, SSC) and green fluorescence (FL1), we performed a shake flask cultivation of the strain producing EcPGA (Pp4) for a microscopic evaluation of the population (Supplementary Figure 7). The strain was cultured in 25 mL BMG with subsequent methanol pulses, and analyzed by flow cytometry and fluorescent microscopy. According to the flow cytometric data (FSC-SSC, FSC-FL1), the division of the population into two sub-populations of different sizes and complexities was less pronounced in a shake flask (Supplementary Figure 7) than in a bioreactor (Supplementary Figure 3). Nevertheless, the population heterogeneity was observed microscopically in the shake flask culture; there were budding cells (two, rarely three connected cells), small oval cells (likely freshly separated cells after division) with weak fluorescence and big round cells with fluorescence of different intensities.
Physiology- and UPR-Related Effects of Production of Different Heterologous Proteins
The concentration (U L−1) of the active enzyme (EcPGA, CaLB and TlXynA) was assessed during fed-batch cultivation (cultivation conditions specified in 3.3) in the centrifuged cultivation medium as well as in the cells (Figure 4). A strong intracellular accumulation of EcPGA was observed, as 50–70% of the total active EcPGA remained in the cells over the whole production phase. In contrast, only a small proportion (2–10%) of the total active CaLB or TlXynA was detected in the cells after 20 h of production. Also, the total protein concentration (g L−1) was assessed in the centrifuged cultivation medium and recalculated to the volume of the culture broth (Supplementary Figure 8). The activities of the extracellular (secreted) enzymes related to the mass of total extracellular protein are shown in Figure 4. In case of CaLB (Figure 4C), the amount of active enzyme per mass of total protein (U g) was more or less stable during the production phase, indicating that the portion of the active CaLB in the secreted protein content was constant. In case of EcPGA (Figures 4A,B) and TlXynA (Figure 4D), the increase of active enzyme per mass of total protein after 40–50 h of production indicated an increased portion of the active enzyme in the extracellular protein content.
According to the flow cytometric data evaluated with PCA, the cell population was heterogeneous and the distribution of the cells into the four sub-populations (defined in chapter Evaluation of the Flow Cytometry Data From Bioreactor Cultivations) changed over the time course of the cultivation process (Figure 6). In the strains producing different recombinant proteins at the specific growth rate with methanol 0.016 h−1 (Figures 6A–C), the amount of the viable smaller and less complex cells increased after the shift to methanol, i.e., the start of the production phase, and was around 30% over the whole production phase. At the same time, the number of the viable bigger cells decreased. In the control non-producing strain (Figure 6D), the number of these smaller and less complex cells began to decrease again after some 20 h of growth on methanol, while the number of the viable larger cells increased proportionally. A decrease in viability was observed in all strains, including the control strain. Around 12% of the population of the control strain was stained with PI at the end of the cultivation, i.e., after 54 h of growth on methanol (Figure 6D), indicating that viability was impaired by long exposure of the cells to methanol. The production of EcPGA did not have any negative effect on cell viability (Figure 6A), since the number of the PI-stained cells was comparable to the control non-producing strain. However, the production of CaLB and TlXynA impaired cell viability compared to the control strain; around 20% of the cells of the CaLB- (Figure 6B) and the TlXynA-producing strains (Figure 6C) had damaged membranes after 54 h of production. The number of damaged cells within the TlXynA-producing strain further increased and was 27% by the end of the process, i.e., after 69 h of production (Figure 6C). The raw flow cytometry data showing the percentage of non-viable (PI-stained) cells are shown in Supplementary Figure 6. It can be seen that in case of TlXynA, the percentage of the cells with impaired viability differed significantly in the two sub-populations of different FSC. While the viability of the “smaller” cells was comparable to the control strain (Supplementary Figure 6A), the viability of the “bigger” cells decreased dramatically (Supplementary Figure 6B).
Figure 6.
Change of cell size and complexity, UPR up-regulation and viability during cultivations of P. pastoris strains producing different recombinant proteins. The P. pastoris strains producing EcPGA (A), CaLB (B), or TlXynA (C), as well as a control non-production strain (D) were cultured under the same cultivation conditions (30°C, pH 5.5), maintaining the specific growth rate of biomass with methanol at 0.016 h−1. Additionally, the P. pastoris strain producing CaLB was cultured at 30°C, pH 5.5 and at a specific growth rate of biomass with methanol of 0.008 h−1 (E) and 0.032 h−1 (F). The four sub-populations identified with the PCA of the flow cytometric data were observed in all the cultivation processes: smaller and less complex viable cells with no UPR up-regulation (red ovals); larger and more complex viable cells with no UPR up-regulation (green ovals); viable cells with up-regulated UPR (black ovals); and cells with an impaired viability (blue ovals).
There was only a slight up-regulation of UPR (around 4% of the population) observed in the non-producing control strain (Figure 6D). In contrast, a significant up-regulation of UPR was observed in all the production strains (Figures 6A–C). In the strain producing and also considerably intracellularly accumulating EcPGA (Figure 4A), the number of cells with up-regulated UPR began to increase steeply right after induction of production (Figure 6A). After ~30 h of production and until the end of the process, up to 60% of the cell population had an up-regulated UPR. Interestingly, in the strains producing CaLB (Figure 6B) and TlXynA (Figure 6C), the maximum portion of cells with up-regulated UPR (35% in case of CaLB production and 26% in case of TlXynA production) was also reached after 30 h of production, although further development was different. The portion of cells with up-regulated UPR was stable over the whole production phase in the CaLB-producing strain, while it began to decrease in the TlXynA-producing strain after 40 h of production and was 14% at the end of cultivation. The stagnation/decrease in the number of cells with an up-regulated UPR might have been caused by lysis of the damaged cells, which was enhanced in the case of production of CaLB and TlXynA, and a consequent release of the intracellular contents, including sfGFP, to the cultivation medium. Alternatively, also a potential loss of the expression cassette (Zhu et al., 2009) might have contributed to the stress relief, i.e., stagnation of the portion of cells with up-regulated UPR.
We further investigated the potential leakage of sfGFP from the cells by measuring the fluorescence of the cultivation medium (extracellular fluorescence) with a microplate fluorimeter. We compared the specific extracellular fluorescence (AU gCDW−1) of the cultivation medium from the processes with the TlXynA-producing strain, the non-producing control strain, and the control strain producing EcPGA, but no sfGFP (Figure 7). For the TlXynA-producing strain, we also measured the fluorescence of the cell lysate (intracellular fluorescence). The specific extracellular fluorescence (AU gCDW−1) in the case of the TlXynA-producing strain began to increase after 15 h of the production phase, and continued increasing until the end of the cultivation. An increase in specific extracellular fluorescence was also observed for the control strains. Therefore, we assume that the increase in extracellular fluorescence was partly caused by the fluorescent background of the culture medium fed into the bioreactor. Nevertheless, after 30 h of production, the specific extracellular fluorescence in the cultivation with the TlXynA-producing strain started to be higher than the specific extracellular fluorescence in cultivations with the control strains. Around the same time point, the number of cells with up-regulated UPR started to decrease, and the number of PI-stained cells in the population exceeded 12% (Figure 6C). The specific intracellular fluorescence measured with the fluorimeter also started to increase after the first 13 h of production, increased steeply until 30 h of production, and then less steeply until 50 h of production. After 50 h of production, it began to decrease. Based on these results, we assume that some of the TlXynA-producing cells were really lysed and releasing sfGFP to the medium. The extracellular sfGFP is not measurable with flow cytometry, which can only detect fluorescence of the cells. Therefore, with our method, we were not able to detect cells with up-regulated UPR and damaged membranes, as a separate cluster.
Figure 7.
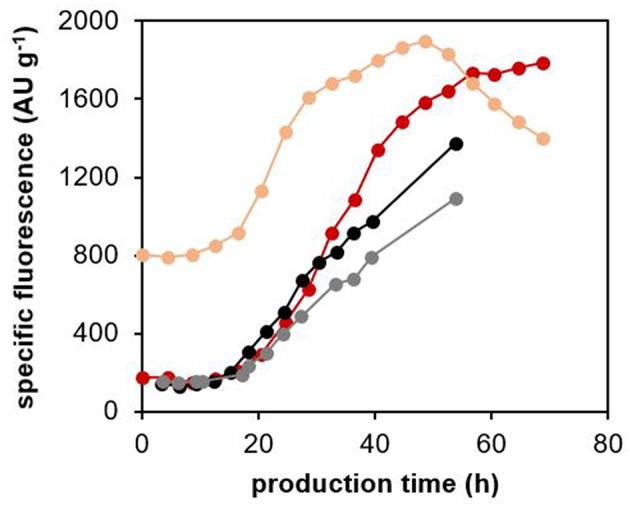
Specific fluorescence of the culture medium and cell lysates of the P. pastoris strain producing TlXynA, and the control strains. The P. pastoris strain producing TlXynA was cultured at 30°C and pH 5.5, maintaining the specific growth rate of biomass with methanol during the production phase at 0.016 h−1. Fluorescence of the centrifuged culture medium and of the cell lysates were measured with a fluorimeter. Specific extracellular fluorescence (dark red) and specific intracellular fluorescence (light red) were calculated from the measured fluorescence (AU) and CDW (g) values. As controls, the specific extracellular fluorescence of the EcPGA-producing strain with no sfGFP production (gray), and the non-producing strain (black) are shown. The control strains were cultured under the same cultivation conditions as the TlXynA-producing strain.
The Influence of Specific Growth Rate on CaLB Production, Physiology and ER-Stress
The P. pastoris strain producing and secreting CaLB was cultured at 30°C and pH 5.5 during fed-batch cultivations with methanol, maintaining the specific growth rate of biomass with methanol at 0.008 h−1, 0.016 h−1 or 0.032 h−1 (Table 1), which corresponded to 15, 30, and 60% of the maximum specific growth rate with methanol, respectively.
The concentration of the product was assessed in the culture broth as well as in the cell extracts, and the specific yield of CaLB was calculated (Figure 8). During any of the cultivations with the three different specific growth rates with methanol, the product did not accumulate significantly inside the cells. During cultivation with a specific growth rate with methanol of 0.016 h−1, the yield of secreted CaLB increased steadily and was around 1,200 U gCDW−1 at the end of the process, i.e., after 70 h of production. In the cultivation with a specific growth rate with methanol of 0.032 h−1, the yield of the secreted CaLB increased less steeply than in the cultivation with μ = 0.016 h−1 and was 600 U gCDW−1 at the end of the process, i.e., after 53 h of production. The lowest yield of the secreted CaLB, 150 U gCDW−1 after 50 h of production, was reached in the cultivation with a specific growth rate with methanol of 0.008 h−1.
Figure 8.
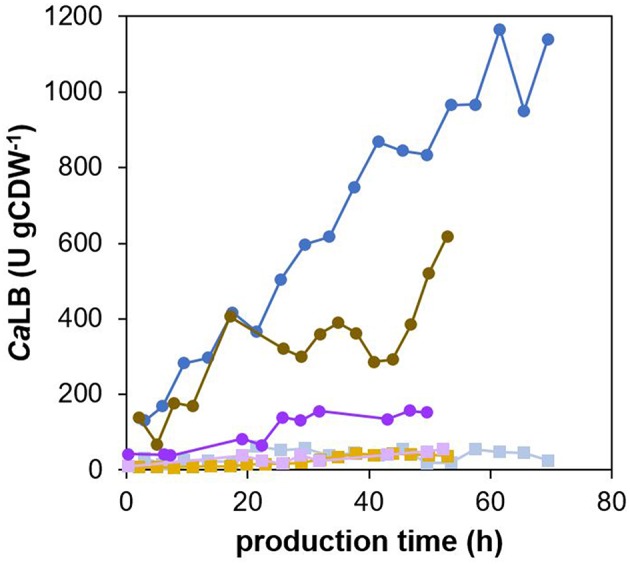
CaLB/biomass yield during cultivations of P. pastoris producing recombinant CaLB under different specific growth rates with methanol. The P. pastoris strain producing CaLB was cultured at 30°C and pH 5.5, maintaining the specific growth rate of biomass with methanol during the production phase at either 0.008 h−1 (purple), 0.016 h−1 (blue), or 0.032 h−1 (brown). The secreted (dark purple/blue/brown circles) as well as the intracellular (light purple/blue/brown squares) CaLB/biomass yield were calculated.
The distribution of the cell population into four sub-populations, as determined by flow cytometry (PCA), differed for the three different specific growth rates with methanol over the time course of the process (Figure 6). The amount of viable smaller and less complex cells during the production phase was significantly higher (between 50 and 70%) at a growth rate of 0.008 h−1 (Figure 6E), than at growth rates of 0.016 h−1 (Figure 6B) or 0.032 h−1 (Figure 6F), where the portion of the viable smaller and less complex cells was 40% or lower during the production phase. The highest portion of cells with damaged membranes (30% after 53 h of production) was observed at μ 0.032 h−1 (Figure 6F); at μ 0.016 h−1, it was around 20% after 53 h of production (Figure 6B), and at μ 0.008 h−1, it was around 16% after 52 h of production (Figure 6E). The greatest impairment to the viability at μ 0.032 h−1 cannot be attributed to a high yield of secreted CaLB (the highest was reached at μ 0.016 h−1), nor to the faster feeding of methanol, since the portion of PI-stained cells in a non-producing control strain cultured at μ 0.032 h−1 did not exceed 13% within a 55 h production phase (Supplementary Figure 9). The number of cells with up-regulated UPR slightly increased over the whole production phase at μ 0.008 h−1 and was 20–25% at the end of the process (Figure 6E). During the process with μ 0.032 h−1, the maximum portion of cells with up-regulated UPR (48%) was reached after 17 h of production (Figure 6F), which was more and earlier, compared to cultivation at μ 0.016 h−1 (Figure 6B). After 17 h of production, the number of the cells belonging to the cluster with up-regulated UPR began to decrease and was 28% at the end of the process, i.e., after 53 h of production.
Discussion
Novel Method of Monitoring Up-Regulation of UPR in P. pastoris
In S. cerevisiae, the UPRE from the KAR2 promoter (Cox et al., 1993), or in Yarrowia lipolytica, the KAR2 promoter (Madzak and Beckerich, 2006) were previously used to control the expression of the lacZ reporter gene. Induction of the UPR was then monitored as the production of β-galactosidase. We applied for the first time a similar approach to monitor the UPR in the yeast P. pastoris. But instead of lacZ, we fused the gene of the fast maturating sfGFP (Pédelacq et al., 2006; Khmelinskii et al., 2012) to the KAR2 promoter (upstream region), to monitor its activity. We showed that the sfGFP signal increased after induction of recombinant protein production (Figure 6; Supplementary Figure 6), which was in accordance with the increase in KAR2 expression, i.e., the UPR up-regulation (Figure 3). Importantly, the sfGFP fluorescence could have been quickly and easily detected with flow cytometry, which enabled a single-cell, at-line and non-invasive analysis of the UPR during fed-batch bioreactor cultivations. Moreover, cell viability, size and complexity were also analyzed simultaneously with flow cytometry.
P. pastoris Population Is Heterogeneous in Terms of Cell Size and Complexity, Up-Regulation of UPR and Cell Viability During Bioreactor Cultivations
Our work provides an interesting insight into the development of a heterogeneous cell population over the time course of a bioreactor cultivation (Figure 6). After the inoculation of the bioreactor, i.e., at the very beginning of the batch phase, the population was homogeneous, formed by larger and more complex cells (higher FSC). Already during the batch phase and further until the start of the production phase with methanol, the population became heterogeneous in terms of cell size and complexity, as the number of smaller and less complex cells (lower FSC) increased. After the start of the production phase, the increasing number of cells with up-regulated UPR was consistent with the decreasing number of viable large and complex cells, assuming that the UPR was being up-regulated mostly in these larger and complex cells. The number of cells with impaired viability increased with the decreasing number of smaller and less complex cells, as well as with the decreasing number of cells with up-regulated UPR. In other words, damaged cells likely arose from smaller and less complex cells, and partly from the large cells with up-regulated UPR. Also, the number of damaged cells increased at different rates and to different extents, depending on the product and specific growth rate with methanol. Cells with an up-regulated UPR and a damaged membrane were not detectable as a separate cluster with our method; as soon as the membrane was damaged, the sfGFP probably leaked out from the cells, so there was no measurable green fluorescence signal in these damaged cells.
The coexistence of P. pastoris cells of different sizes, especially during methanol feeding, was reported previously (Hohenblum et al., 2003). It was also shown that the non-producing cells were larger (as indicated by the mean FSC with flow cytometry) than the cells producing the recombinant antibody Fab fragment (Dragosits et al., 2010). According to another work (Aw et al., 2017), P. pastoris strains secreting a higher amount of a recombinant protein were shown to have larger cells (as determined by flow cytometry) than strains producing the same product, but lower titers. In our work, the median FSC during the production phase with methanol was comparable in case of the non-producing control strain (median FSC 14.0) and the strain producing EcPGA (median FSC 15.3). The median FSC of the strains producing CaLB and TlXynA was lower (9.1 and 9.7, respectively). Moreover, in the case of the strain producing CaLB, we observed a correlation between FSC and specific growth rate with methanol. The average FSC value (referring to cell size) over the production phase was 6.6 at the lowest growth rate of 0.008 h−1. At growth rate 0.016 h−1, the average FSC was 9.1 and at the highest growth rate 0.032 h−1, the average FSC was 12.6. This trend was, however, not observed in the control strain producing no recombinant protein; the cell sizes were comparable at the specific growth rates with methanol of 0.016 h−1 (FSC 14.0) and 0.032 h−1 (FSC 12.1). In S. cerevisiae, several studies [summarized by Kacmar et al. (2006)] showed that cell size increased with an increasing specific growth rate. However, the increase in cell size was shown to be caused by the production of ethanol at higher growth rates, rather than by a higher specific growth rate (Kacmar et al., 2006).
The heterogeneity in terms of viability of the P. pastoris population producing a recombinant protein has been reported in many works (Hohenblum et al., 2003; Jahic et al., 2003; Hyka et al., 2010; Rebnegger et al., 2014, 2016; Zhong et al., 2014; Wang et al., 2016; Aw et al., 2017; Madjid Ansari et al., 2017; Reséndiz-Cardiel et al., 2017). To our best knowledge, the UPR has not been analyzed at the single-cell level in P. pastoris until now.
In our work, we did not measure the secretion and intracellular accumulation of the recombinant proteins (EcPGA, CaLB, TlXynA) at a single-cell resolution, as previously shown using microengraving (Love et al., 2010, 2012) or tagging the protein of interest with eGFP (Broger et al., 2011). It was reported that the population of P. pastoris cells constitutively producing different recombinant proteins (eGFP, glycosylated/aglycosylated human Fc fragment) was heterogeneous in terms of protein secretion and growth rate. Independently from the type of recombinant protein, three sub-populations with regard to protein secretion were identified: a large subpopulation (35%) that did not secrete the protein significantly but was viable, a subpopulation secreting the protein consistently, and a subpopulation with a changeable rate of protein secretion (Love et al., 2010, 2012). Heterogeneity in terms of protein production was also reported in the case of P. pastoris producing human membrane protein tagged with eGFP (Broger et al., 2011). The co-existence of the sub-populations with different fluorescence intensities indicated varying protein productivity, which was likely caused by varying copy numbers of the recombinant gene in the cells (Cregg et al., 2000). Also non-producing cells were identified within the population (Broger et al., 2011). Combining our method for the single-cell detection of cell size/complexity, UPR and viability with single-cell detection of the recombinant protein, or sorting the cells by FC according to their properties and subsequently measuring the product in the sorted subpopulations, it would be possible to relate the cell types characterized in this work (smaller vs. larger cells, cells with up-regulated UPR, cells with damaged membranes) to their secretion phenotype.
Production of Recombinant Proteins in P. pastoris Up-Regulates UPR and Impairs Viability
All three recombinant proteins used in this work, i.e., EcPGA, CaLB, and TlXynA, up-regulated the UPR in P. pastoris, though to different extent, depending on the type of the recombinant protein (EcPGA, CaLB, TlXynA, no protein), and, in the case of CaLB, on the specific growth rate with methanol (0.008 h−1, 0.016 h−1, 0.032 h−1). Interestingly, the maximum number of cells with an up-regulated UPR was always reached after 30 h of production, independent of the product, but probably depending on the specific growth rate with methanol.
The majority of studies report UPR up-regulation by (over)production of a recombinant protein (Hohenblum et al., 2004; Gasser et al., 2007; Resina et al., 2007; Khatri et al., 2011; Sjöblom et al., 2012; Hesketh et al., 2013; Vogl et al., 2014; Zhong et al., 2014; Wang et al., 2017; Yu et al., 2017). Only the induced production of secretory insulin precursor in P. pastoris was reported to be accompanied by a decrease in the amount of Kar2p in the cells upon methanol shift production (Vanz et al., 2014). This phenomenon was, however, recently explained not by a down-regulation of UPR, but by secretion of Kar2p along with the recombinant protein to the medium and/or by an enhanced autophagy of the ER, resulting in an extracellular release of Kar2p (Roth et al., 2018).
Generally, up-regulation of UPR may be more significant in a fed-batch mode than in chemostat cultures (Zahrl et al., 2017). Up-regulation of UPR was shown to correlate with the intracellular accumulation of human trypsinogen (Hohenblum et al., 2004) or interleukin (Zhong et al., 2014). In contrast, in the case of partial intracellular accumulation of the mucin-type protein fused with GFP, the ER folding stress was low; however there was a secretory bottle-neck in the Golgi system (Sjöblom et al., 2012). In another work, the UPR was reported to be up-regulated to a different extent in P. pastoris clones producing human serum albumin (HSA), which all contained only one copy of the gene coding for HSA (Aw et al., 2017); there was no clear correlation between up-regulation of the UPR, and product titers, but the ERAD was only up-regulated in clones with high secretion of HSA. The UPR was also shown to be down-regulated at lower specific growth rates (Rebnegger et al., 2014). In our work, the increasing specific growth rate with methanol resulted in a faster and stronger up-regulation of UPR.
In this work, the up-regulation was most significant in the case of EcPGA (60% of the EcPGA-producing cells exhibited up-regulated UPR), which strongly accumulated in the cells (up to 50–70% of the total active EcPGA). As already discussed in the work of Marešová et al. (2017), the reason for poor secretion of EcPGA might be its incorrect maturation in P. pastoris cells, which results in ER stress. A co-expression of E. coli chaperone genes might improve the secretion of proteins of bacterial origin (Summpunn et al., 2018). The recombinant CaLB and TlXynA in our work did not accumulate significantly inside the cells, yet we observed an up-regulation of the UPR. This was probably caused by the overexpression of the recombinant genes from the AOX1 promoter, which resulted in a high load of protein in the ER. Since the post-translational modifications of TlXynA involve formation of disulfide bonds (Gruber et al., 1998; Damaso et al., 2003), and in the case of CaLB, also glycosylation (Uppenberg et al., 1994), we believe the capacity of the ER was exceeded. The up-regulation of UPR by the production of recombinant xylanase A in P. pastoris was reported previously (Lin et al., 2013); compared to a control non-producing strain, the fold change in KAR2 expression analyzed by qPCR was ~0.5 in a strain carrying one copy of the xylanase gene, and 1.9 in a strain carrying four copies of the xylanase gene. No ERAD up-regulation was observed, indicating that the xylanase was not degraded (Lin et al., 2013). Detectable amounts of Kar2p were also observed in the P. pastoris strain producing CaLB (Samuel et al., 2013), but there was no comparison made to a non-producing strain. Overall, our results indicate that the up-regulation of UPR was not exclusively linked to poor secretion of the recombinant protein. Additionally, there was also no clear correlation between the intensity of UPR caused by the different proteins, and the mass of the secreted total protein (g) (Supplementary Figure 8). There is a general demand for more detailed characterization of the secretory machinery and the UPR in P. pastoris, information that is currently still derived from other yeasts (Puxbaum et al., 2015).
The viability of the cells was impaired by the production of CaLB and TlXynA, as the number of PI-stained cells in these strains was higher than in the non-producing control strain. In contrast to CaLB and TlXynA, the production of EcPGA in our work did not enhance cellular damage, compared to the non-producing control strain. The impaired viability of P. pastoris resulting from production of recombinant protein is commonly observed, although to different extents, depending on the recombinant protein and cultivation conditions. For example, in the case of the production of recombinant human trypsinogen, 65% of cells were non-viable after ~135 h of production with methanol (Hohenblum et al., 2003). The production of porcine trypsinogen at pH 4 and at a high cell density in fed-batch culture resulted in more than 80% of damaged cells (Hyka et al., 2010). High viability was observed at very low, almost zero, specific growth rates (Rebnegger et al., 2016). In another study, however, there was no clear correlation between specific growth rates, viability and product yield; at the lowest specific growth rate (0.015 h−1), the viability was 94%, while at the higher specific growth rates, the number of dead cells was even lower (Rebnegger et al., 2014). In our work, the increasing specific growth rate with methanol resulted in a larger fraction of damaged cells, but the highest specific yield of CaLB was reached at the mid value within the range of the tested specific growth rates with methanol, i.e., at 0.016 h−1.
Conclusions
Production and secretion of three different recombinant proteins (EcPGA, CaLB, TlXynA) by P. pastoris was investigated along with up-regulation of the UPR and cell viability, which were assessed at the single-cell level in living cells and at-line with flow cytometry. In all three strains, as well as in a non-producing control strain, which were all cultured under the same cultivation conditions, a heterogeneous population consisting of four subpopulations developed after the induction of protein production. The distribution of the population into four fractions differed over the time course of the cultivation process and depended on the recombinant protein and the specific growth rate. Up-regulation of the UPR was especially strong when the recombinant protein was not properly secreted (EcPGA), but was not linked exclusively to the intracellular accumulation. We observed no correlation between up-regulation of UPR and impaired viability, and also no clear correlation between viability and protein production (CaLB). We believe these results emphasize the importance of a complex and systematic understanding of the biotechnological process, with a single-cell analysis of the production population, in order to enhance productivity of the bioprocess. We developed a reporter system for the UPR, which enables fast and easy monitoring of the UPR at the single-cell level and in a non-invasive manner. This method could be adapted to a broad range of biotechnologically important production hosts and also, with no necessary adaptation, could be used at the screening scale. By understanding the relationship between protein production/secretion and the tuning of the UPR, this monitoring system based on fluorescence measurement might be utilized in a feedback control of a bioprocess.
Author Contributions
HR designed and conducted the experiments and wrote the manuscript. IZ created the program for the analysis of the flow cytometric data. MB and AW participated in the construction of the recombinant strains. PM participated in performing the bioreactor experiments and analyzing the data from the cultivation processes. DM presented this work at the conference Microbial Stress: from Systems to Molecules and Back in Kinsale, Ireland. ZK designed the expression cassette for monitoring the unfolded protein response and supervised the work. AG, KM, and KK supervised the work.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The major part of this research was performed within the project Physiological response of Pichia pastoris to production of a heterologous protein (PHYRE) supported by the Swiss Government Excellence Scholarship. The work was also financially supported from specific university research (MSMT No 20-SVV/2017). Big thanks belongs to Marcel Straumann, Chiara Braun, Sonja Weichart, and Dr. Lukas Neutsch (all Zurich University of Applied Sciences ZHAW, Wädenswil, CH) for assisting with the bioreactor cultivations. The authors also thank Dr. Helena Marešová (Institute of Microbiology of the Czech Academy of Sciences, Czech Republic) for providing the plasmid carrying the EcPGA, Prof. Dr. Michael Knop (Zentrum für Molekulare Biologie der Universität Heidelberg, Heidelberg, DE) for providing the plasmid pMaM60 and bisy e.U., Hofstätten for providing the plasmids pBSY3S1Z_CaLB-WT, pBSYAOXsec_blunt, and pBSYAOX_blunt. Further thanks goes to Prof. Dr. John Brooker for English proofreading.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2019.00335/full#supplementary-material
References
- Ahmad M., Hirz M., Pichler H., Schwab H. (2014). Protein expression in Pichia pastoris: recent achievements and perspectives for heterologous protein production. Appl. Microbiol. Biotechnol. 98, 5301–5317. 10.1007/s00253-014-5732-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alber A. B., Paquet E. R., Biserni M., Naef F., Suter D. M. (2018). Single live cell monitoring of protein turnover reveals intercellular variability and cell-cycle dependence of degradation rates. Mol. Cell. 71, 1079–1091. 10.1016/j.molcel.2018.07.023 [DOI] [PubMed] [Google Scholar]
- Aw R., Barton G. R., Leak D. J. (2017). Insights into the prevalence and underlying causes of clonal variation through transcriptomic analysis in Pichia pastoris. Appl. Microbiol. Biotechnol. 101, 5045–5058. 10.1007/s00253-017-8317-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broger T., Odermatt R. P., Huber P., Sonnleitner B. (2011). Real-time on-line flow cytometry for bioprocess monitoring. J. Biotechnol. 154, 240–247. 10.1016/j.jbiotec.2011.05.003 [DOI] [PubMed] [Google Scholar]
- Cereghino J. L., Cregg J. M. (2000). Heterologous protein expression in the methylotrophic yeast Pichia pastoris. FEMS Microbiol. Rev. 24, 45–66. 10.1111/j.1574-6976.2000.tb00532.x [DOI] [PubMed] [Google Scholar]
- Cox J. S., Shamu C. E., Walter P. (1993). Transcriptional induction of genes encoding endoplasmic reticulum resident proteins requires a transmembrane protein kinase. Cell 73, 1197–1206. 10.1016/0092-8674(93)90648-A [DOI] [PubMed] [Google Scholar]
- Cregg J. M., Cereghino J. L., Shi J., Higgins D. R. (2000). Recombinant protein expression in Pichia pastoris. Mol. Biotechnol. 16, 23–52. 10.1385/MB:16:1:23 [DOI] [PubMed] [Google Scholar]
- Damaso M. C., Almeida M. S., Kurtenbach E., Martins O. B., Pereira N, Jr, Andrade C. M., et al. (2003). Optimized expression of a thermostable xylanase from Thermomyces lanuginosus in Pichia pastoris. Appl. Env. Microbiol. 69, 6064–6072. 10.1128/AEM.69.10.6064-6072.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragosits M., Stadlmann J., Graf A., Gasser B., Maurer M., Sauer M., et al. (2010). The response to unfolded protein is involved in osmotolerance of Pichia pastoris. BMC Genomics 11:207. 10.1186/1471-2164-11-207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek J., Benedix J., Cappel S., Greiner M., Jalal C., Müller L., et al. (2009). Functions and pathologies of BiP and its interaction partners. Cell. Mol. Life Sci. 66, 1556–1569. 10.1007/s00018-009-8745-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards-Jones B., Aw R., Barton G. R., Tredwell G. D., Bundy J. G., Leak D. J. (2015). Translational arrest due to cytoplasmic redox stress delays adaptation to growth on methanol and heterologous protein expression in a typical fed-batch culture of Pichia pastoris. PLoS ONE 10:e0119637. 10.1371/journal.pone.0119637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao B., Wang X., Shen Y. (2006). Studies on characters of immobilizing penicillin G acylase on a novel composite support PEI/SiO2. Biochem. Eng. J. 28, 140–147. 10.1016/j.bej.2005.10.007 [DOI] [Google Scholar]
- Gasser B., Maurer M., Rautio J., Sauer M., Bhattacharyya A., Saloheimo M., et al. (2007). Monitoring of transcriptional regulation in Pichia pastoris under protein production conditions. BMC Genomics 8:179. 10.1186/1471-2164-8-179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf A., Gasser B., Dragosits M., Sauer M., Leparc G. G., Tüchler T., et al. (2008). Novel insights into the unfolded protein response using Pichia pastoris specific DNA microarrays. BMC Genomics 9:390. 10.1186/1471-2164-9-390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber K., Klintschar G., Hayn M., Schlacher A., Steiner W., Kratky C. (1998). Thermophilic xylanase from thermomyces lanuginosus: high-resolution X- ray structure and modeling studies. Biochemistry 37, 13475–13485. 10.1021/bi980864l [DOI] [PubMed] [Google Scholar]
- Guerfal M., Ryckaert S., Jacobs P. P., Ameloot P., Van Craenenbroeck K., Derycke R., et al. (2010). The HAC1 gene from Pichia pastoris: characterization and effect of its overexpression on the production of secreted, surface displayed and membrane proteins. Microb. Cell Fact. 9:49. 10.1186/1475-2859-9-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesketh A. R., Castrillo J. I., Sawyer T., Archer D. B., Oliver S. G. (2013). Investigating the physiological response of Pichia (Komagataella) pastoris GS115 to the heterologous expression of misfolded proteins using chemostat cultures. Appl. Microbiol. Biotechnol. 97, 9747–9762. 10.1007/s00253-013-5186-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohenblum H., Borth N., Mattanovich D. (2003). Assessing viability and cell-associated product of recombinant protein producing Pichia pastoris with flow cytometry. J. Biotechnol. 102, 281–290. 10.1016/S0168-1656(03)00049-X [DOI] [PubMed] [Google Scholar]
- Hohenblum H., Gasser B., Maurer M., Borth N., Mattanovich D. (2004). Effects of gene dosage, promoters, and substrates on unfolded protein stress of recombinant Pichia pastoris. Biotechnol. Bioeng. 85, 367–375. 10.1002/bit.10904 [DOI] [PubMed] [Google Scholar]
- Hyka P., Züllig T., Ruth C., Looser V., Meier C., Klein J., et al. (2010). Combined use of fluorescent dyes and flow cytometry to quantify the physiological state of Pichia pastoris during the production of heterologous proteins in high-cell-density fed-batch cultures. Appl. Env. Microbiol. 76, 4486–4496. 10.1128/AEM.02475-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahic M., Wallberg F., Bollok M., Garcia P., Enfors S. O. (2003). Temperature limited fed-batch technique for control of proteolysis in Pichia pastoris bioreactor cultures. Microb. Cell Fact. 2:6. 10.1186/1475-2859-2-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juturu V., Wu J. C. (2018). Heterologous protein expression in Pichia pastoris: latest research progress and applications. ChemBioChem 19, 7–21. 10.1002/cbic.201700460 [DOI] [PubMed] [Google Scholar]
- Kacmar J., Gilbert A., Cockrell J., Srienc F. (2006). The cytostat: a new way to study cell physiology in a precisely defined environment. J. Biotechnol. 126, 163–72. 10.1016/j.jbiotec.2006.04.015 [DOI] [PubMed] [Google Scholar]
- Khatri N. K., Gocke D., Trentmann O., Neubauer P., Hoffmann F. (2011). Single-chain antibody fragment production in Pichia pastoris: benefits of prolonged pre-induction glycerol feeding. Biotechnol. J. 6, 452–462. 10.1002/biot.201000193 [DOI] [PubMed] [Google Scholar]
- Khmelinskii A., Keller P. J., Bartosik A., Meurer M., Barry J. D., Mardin B. R., et al. (2012). Tandem fluorescent protein timers for in vivo analysis of protein dynamics. Nat. Biotechnol. 30, 708–714. 10.1038/nbt.2281 [DOI] [PubMed] [Google Scholar]
- Krainer F. W., Dietzsch C., Hajek T., Herwig C., Spadiut O., Glieder A. (2012). Recombinant protein expression in Pichia pastoris strains with an engineered methanol utilization pathway. Microb. Cell Fact. 11:22. 10.1186/1475-2859-11-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lajoie P., Moir R. D., Willis I. M., Snapp E. L. (2012). Kar2p availability defines distinct forms of endoplasmic reticulum stress in living cells. Mol. Biol. Cell 23, 955–964. 10.1091/mbc.e11-12-0995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X. Q., Liang S. L., Han S. Y., Zheng S. P., Ye Y. R., Lin Y. (2013). Quantitative iTRAQ LC-MS/MS proteomics reveals the cellular response to heterologous protein overexpression and the regulation of HAC1 in Pichia pastoris. J. Proteomics 91, 58–72. 10.1016/j.jprot.2013.06.031 [DOI] [PubMed] [Google Scholar]
- Lin-Cereghino J., Wong W. W., Xiong S., Giang W., Luong L. T., Vu J., et al. (2005). Condensed protocol for competent cell preparation and transformation of the methylotrophic yeast Pichia pastoris. Biotechniques 38, 44–48. 10.2144/05381BM04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love K. R., Panagiotou V., Jiang B., Stadheim T. A., Love J. C. (2010). Integrated single-cell analysis shows Pichia pastoris secretes protein stochastically. Biotechnol. Bioeng. 106, 319–325. 10.1002/bit.22688 [DOI] [PubMed] [Google Scholar]
- Love K. R., Politano T. J., Panagiotou V., Jiang B., Stadheim T. A., Love J. C. (2012). Systematic single-cell analysis of Pichia pastoris reveals secretory capacity limits productivity. PLoS ONE 7:e37915. 10.1371/journal.pone.0037915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madjid Ansari A., Majidzadeh -A K, Darvishi B., Sanati H., Farahmand L., Norouzian D., et al. (2017). Extremely low frequency magnetic field enhances glucose oxidase expression in Pichia pastoris GS115. Enzyme Microb. Technol. 98, 67–75. 10.1016/j.enzmictec.2016.12.011 [DOI] [PubMed] [Google Scholar]
- Madzak C., Beckerich J.-M. (2006). A sensor of the unfolded protein response to study the stress induced in Yarrowia lipolytica strains by the production of heterologous proteins. Microb. Cell Fact. 5:P5 10.1186/1475-2859-5-S1-P5 [DOI] [Google Scholar]
- Marešová H., Palyzová A., Plačková M., Grulich M., Rajasekar V. W., Štěpánek V., et al. (2017). Potential of Pichia pastoris for the production of industrial penicillin G acylase. Folia Microbiol. (Praha). 62, 417–424. 10.1007/s12223-017-0512-0 [DOI] [PubMed] [Google Scholar]
- Mattanovich D., Gasser B., Hohenblum H., Sauer M. (2004). Stress in recombinant protein producing yeasts. J. Biotechnol. 113, 121–135. 10.1016/j.jbiotec.2004.04.035 [DOI] [PubMed] [Google Scholar]
- Meehl M. A., Stadheim T. A. (2014). Biopharmaceutical discovery and production in yeast. Curr. Opin. Biotechnol. 30, 120–127. 10.1016/j.copbio.2014.06.007 [DOI] [PubMed] [Google Scholar]
- Mellitzer A., Glieder A., Weis R., Reisinger C., Flicker K. (2012a). Sensitive high-throughput screening for the detection of reducing sugars. Biotechnol. J. 7, 155–162. 10.1002/biot.201100001 [DOI] [PubMed] [Google Scholar]
- Mellitzer A., Weis R., Glieder A., Flicker K. (2012b). Expression of lignocellulolytic enzymes in Pichia pastoris. Microb. Cell Fact. 11:61. 10.1186/1475-2859-11-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori K., Kawahara T., Yoshida H., Yanagi H., Yura T. (1996). Signalling from endoplasmic reticulum to nucleus: transcription factor with a basic-leucine zipper motif is required for the unfolded protein-response pathway. Genes Cells 1, 803–817. 10.1046/j.1365-2443.1996.d01-274.x [DOI] [PubMed] [Google Scholar]
- Mori K., Ogawa N., Kawahara T., Yanagi H., Yura T. (1998). Palindrome with spacer of one nucleotide is characteristic of the cis-acting unfolded protein response element in Saccharomyces cerevisiae. J. Biol. Chem. 273, 9912–9920. 10.1074/jbc.273.16.9912 [DOI] [PubMed] [Google Scholar]
- Pédelacq J. D., Cabantous S., Tran T., Terwilliger T. C., Waldo G. S. (2006). Engineering and characterization of a superfolder green fluorescent protein. Nat. Biotechnol. 24, 79–88. 10.1038/nbt1172 [DOI] [PubMed] [Google Scholar]
- Pfeffer M., Maurer M., Köllensperger G., Hann S., Graf A. B., Mattanovich D. (2011). Modeling and measuring intracellular fluxes of secreted recombinant protein in Pichia pastoris with a novel 34S labeling procedure. Microb. Cell Fact. 10:47. 10.1186/1475-2859-10-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potgieter T. I., Kersey S. D., Mallem M. R., Nylen A. C., d'Anjou M. (2010). Antibody expression kinetics in glycoengineered Pichia pastoris. Biotechnol. Bioeng. 106, 918–927. 10.1002/bit.22756 [DOI] [PubMed] [Google Scholar]
- Puxbaum V., Mattanovich D., Gasser B. (2015). Quo vadis? The challenges of recombinant protein folding and secretion in Pichia pastoris. Appl. Microbiol. Biotechnol. 99, 2925–2938. 10.1007/s00253-015-6470-z [DOI] [PubMed] [Google Scholar]
- Rebnegger C., Graf A. B., Valli M., Steiger M. G., Gasser B., Maurer M., et al. (2014). in Pichia pastoris, growth rate regulates protein synthesis and secretion, mating and stress response. Biotechnol. J. 9, 511–525. 10.1002/biot.201300334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebnegger C., Vos T., Graf A. B., Valli M., Pronk J. T., Daran-Lapujade P., et al. (2016). Pichia pastoris exhibits high viability and low maintenance-energy requirement at near-zero specific growth rates. Appl. Environ. Microbiol. 82, 4570–4583. 10.1128/AEM.00638-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reséndiz-Cardiel G., Arroyo R., Ortega-López J. (2017). Expression of the enzymatically active legumain-like cysteine proteinase TvLEGU-1 of Trichomonas vaginalis in Pichia pastoris. Protein Expr. Purif. 134, 104–113. 10.1016/j.pep.2017.04.007 [DOI] [PubMed] [Google Scholar]
- Resina D., Bollók M., Khatri N. K., Valero F., Neubauer P., Ferrer P. (2007). Transcriptional response of P-pastoris in fed-batch cultivations to Rhizopus oryzae lipase production reveals UPR induction. Microb. Cell Fact. 6:21. 10.1186/1475-2859-6-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth G., Vanz A. L., Lünsdorf H., Nimtz M., Rinas U. (2018). Fate of the UPR marker protein Kar2/Bip and autophagic processes in fed-batch cultures of secretory insulin precursor producing Pichia pastoris. Microb. Cell Fact. 17:123. 10.1186/s12934-018-0970-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel P., Prasanna Vadhana A. K., Kamatchi R., Antony A., Meenakshisundaram S. (2013). Effect of molecular chaperones on the expression of Candida antarctica lipase B in Pichia pastoris. Microbiol. Res. 168, 615–620. 10.1016/j.micres.2013.06.007 [DOI] [PubMed] [Google Scholar]
- Sjöblom M., Lindberg L., Holgersson J., Rova U. (2012). Secretion and expression dynamics of a GFP-tagged mucin-type fusion protein in high cell density Pichia pastoris bioreactor cultivations. Adv. Biosci. Biotechnol. 3, 238–248. 10.4236/abb.2012.33033 [DOI] [Google Scholar]
- Sobotková L., Štěpánek V., Plháčková K., Kyslík P. (1996). Development of a high-expression system for penicillin G acylase based on the recombinant Escherichia coli strain RE3(pKA18). Enzyme Microb. Technol. 19, 389–397. 10.1016/S0141-0229(96)00052-X [DOI] [Google Scholar]
- Summpunn P., Jomrit J., Panbangred W. (2018). Improvement of extracellular bacterial protein production in Pichia pastoris by co-expression of endoplasmic reticulum residing GroEL–GroES. J. Biosci. Bioeng. 125, 268–274. 10.1016/j.jbiosc.2017.09.007 [DOI] [PubMed] [Google Scholar]
- Theron C. W., Berrios J., Delvigne F., Fickers P. (2018). Integrating metabolic modeling and population heterogeneity analysis into optimizing recombinant protein production by Komagataella (Pichia) pastoris. Appl. Microbiol. Biotechnol. 102, 63–80. 10.1007/s00253-017-8612-y [DOI] [PubMed] [Google Scholar]
- Tredwell G. D., Aw R., Edwards-Jones B., Leak D. J., Bundy J. G. (2017). Rapid screening of cellular stress responses in recombinant Pichia pastoris strains using metabolite profiling. J. Ind. Microbiol. Biotechnol. 44, 413–417. 10.1007/s10295-017-1904-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uppenberg J., Hansen M. T., Patkar S., Jones T. A. (1994). The sequence, crystal structure determination and refinement of two crystal forms of lipase B from Candida antarctica. Structure 2, 293–308. 10.1016/S0969-2126(00)00031-9 [DOI] [PubMed] [Google Scholar]
- Vanz A. L., Nimtz M., Rinas U. (2014). Decrease of UPR- and ERAD-related proteins in Pichia pastoris during methanol-induced secretory insulin precursor production in controlled fed-batch cultures. Microb. Cell Fact. 13:10. 10.1186/1475-2859-13-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogl T., Thallinger G. G., Zellnig G., Drew D., Cregg J. M., Glieder A., et al. (2014). Towards improved membrane protein production in Pichia pastoris: general and specific transcriptional response to membrane protein overexpression. N. Biotechnol. 31, 538–552. 10.1016/j.nbt.2014.02.009 [DOI] [PubMed] [Google Scholar]
- Wang J., Li Y., Liu D. (2016). Improved production of Aspergillus usamii endo-β-1,4-Xylanase in Pichia pastoris via combined strategies. Biomed Res. Int. 2016:3265895. 10.1155/2016/3265895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. D., Jiang T., Yu X. W., Xu Y. (2017). Effects of UPR and ERAD pathway on the prolyl endopeptidase production in Pichia pastoris by controlling of nitrogen source. J. Ind. Microbiol. Biotechnol. 44, 1053–1063. 10.1007/s10295-017-1938-8 [DOI] [PubMed] [Google Scholar]
- Weis R., Luiten R., Skranc W., Schwab H., Wubbolts M., Glieder A. (2004). Reliable high-throughput screening with Pichia pastoris by limiting yeast cell death phenomena. FEMS Yeast Res. 5, 179–189. 10.1016/j.femsyr.2004.06.016 [DOI] [PubMed] [Google Scholar]
- Yu X. W., Sun W. H., Wang Y. Z., Xu Y. (2017). Identification of novel factors enhancing recombinant protein production in multi-copy Komagataella phaffii based on transcriptomic analysis of overexpression effects. Sci. Rep. 7:16249. 10.1038/s41598-017-16577-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahrl R. J., Mattanovich D., Gasser B. (2018). The impact of ERAD on recombinant protein secretion in pichia pastoris (Syn komagataella spp.). Microbiol. 164, 453–463. 10.1099/mic.0.000630 [DOI] [PubMed] [Google Scholar]
- Zahrl R. J., Peña D. A., Mattanovich D., Gasser B. (2017). Systems biotechnology for protein production in Pichia pastoris. FEMS Yeast Res. 17, 1–15. 10.1093/femsyr/fox068 [DOI] [PubMed] [Google Scholar]
- Zhong Y., Yang L., Guo Y., Fang F., Wang D., Li R., et al. (2014). High-temperature cultivation of recombinant Pichia pastoris increases endoplasmic reticulum stress and decreases production of human interleukin-10. Microb. Cell Fact. 13:163. 10.1186/s12934-014-0163-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu T., Guo M., Sun C., Qian J., Zhuang Y., Chu J., et al. (2009). A systematical investigation on the genetic stability of multi-copy Pichia pastoris strains. Biotechnol. Lett. 31, 679–684. 10.1007/s10529-009-9917-4 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.