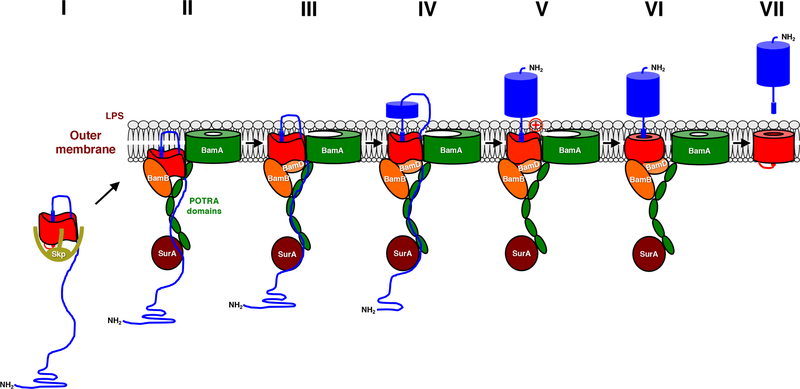
FIG 2.
Model for the assembly of a classical autotransporter. Available evidence suggests that the β barrel domain (red) begins to fold in the periplasm (step I) and incorporates the C terminus of the passenger domain (blue) in a hairpin conformation. At this stage the β barrel domain interacts with the molecular chaperone Skp. The partially folded β barrel domain is then targeted to the OM where it binds to BamA, BamB and BamD in a stereospecific fashion (step II). The surface exposure of the passenger domain and the initiation of translocation requires an additional assembly step in which the β barrel domain moves into the membrane (step III). Both autotransporter and BamA β barrels are in an open conformation at this stage. Translocation involves the progressive movement of passenger domain segments from the chaperone SurA to the POTRA domains of BamA to the transport channel and is driven at least in part by vectorial folding (step IV). Following the completion of translocation the hairpin is resolved (step V), and an unusual lipid-facing basic or large polar residue found in at least a subset of autotransporters facilitates the completion of β barrel domain assembly (step VI). The β barrel domain is then released from the Bam complex and, in some cases, the two domains are separated by an intrabarrel cleavage or an extrabarrel cleavage mediated by a trans-acting protease (step VII). In E. coli the Bam complex contains five subunits, but BamC and BamE have been omitted for clarity. Modified from Molecular Microbiology (58, 101) with the permission of the publisher.