Abstract
Neuroimaging plays a pivotal role in the care of patients with infiltrating gliomas, in whom imaging changes are often the first indications of tumor response or progression. Unfortunately, evaluation of glioma response is often not straightforward, even for experienced radiologists. Post-surgical or radiation-related changes may mimic the appearance of disease progression, while medications such as corticosteroids and antiangiogenic agents may mimic tumor response without truly arresting tumor growth or improving patient survival. Immunotherapy response can result in inflammatory changes which manifest as progressively increasing tumor enhancement and edema over months. Many of these pitfalls can be minimized or avoided altogether by the use of modern brain tumor response criteria, while others will require new imaging tools before they can be fully addressed. Advanced MRI methods and novel positron emission tomography (PET) agents are proving important for this purpose, and their role will undoubtedly continue to grow in the future.
Introduction
Radiologists play a fundamental role in guiding the care of patients with infiltrating glioma, as imaging progression is often the first evidence of tumor progression, preceding any clinical signs of tumor growth. As such, patients undergo frequent imaging and decisions regarding whether to continue or change therapy are heavily influenced by the results of this imaging. Unfortunately, the radiographic findings of tumor progression are often ambiguous. Radiologists can facilitate optimal patient care by being aware of common mimics of tumor progression and response, and using this information to make confident diagnoses of progression or response when possible, or suggesting appropriate follow-up or additional testing when a definitive conclusion is not possible. Much of the current literature concerning issues in glioma imaging deals with selected imaging pitfalls in isolation or offers potential solutions aimed as use in clinical trials. We will review classic and novel pitfalls in glioma response assessment with an emphasis on routine clinical practice and discuss emerging imaging tools that may allow more specific diagnosis of tumor progression in the near future.
Importance of accurate diagnosis of progression
Infiltrating gliomas differ significantly in aggressiveness and response to therapy. The median survival after diagnosis of glioblastoma is less than 2 years, while patients with low-grade oligodendroglioma may live well in excess of a decade.1,2 In both of these scenarios, patients receive first-line therapy and are then observed until progression, at which time second-line therapy options are considered. The interval between imaging may be as short as 2 or 3 months after initial therapy, or as infrequent as annually in low-grade tumors with demonstrated stability on previous imaging studies. While imaging is only one component of response assessment, considered along with clinical elements such as physical examination3 and corticosteroid use by the treating clinician or tumor board, results of imaging are often given significant weight by patients and clinicians.
If mimics of glioma progression occur during first-line therapy and are mistaken for true progression, they may lead to the erroneous discontinuation of effective therapy. If this occurs, patients may not receive the full benefit of first-line therapy. While there are no direct data measuring the survival impact of premature discontinuation of first-line therapy, the survival benefits conferred by the first-line treatments for both low-grade and high-grade glioma exceed the benefits of any currently available salvage therapies.
Glioma progression mimics are also a significant issue for patients treated for recurrent disease. As the number of effective salvage glioma chemotherapeutic options is quite limited, not using each to its full capacity increases the likelihood that a patient will exhaust available treatment options early in the course of disease. Further, salvage treatments with surgical resection or repeat radiation therapy may be considered. While entirely appropriate in the correct clinical circumstances, both of these treatment options may result in morbidity, and in the absence of true progression the potential risks are not matched by any clinical benefit. Aside from the direct impact on the patient, incorrect assessment of tumor progression in patients enrolled in clinical trials has the potential to confound interpretation of the trial results, especially for trials in which progression-free survival is the primary endpoint.
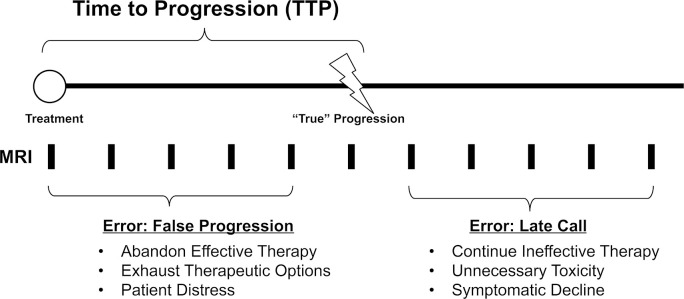
Although the discussion above may seem to argue for a conservative approach to diagnosing tumor progression, delayed diagnosis of progression can also be harmful to patients. Indeed, the entire point of glioma surveillance imaging is to identify early progression, allowing a change in therapy and preventing or delaying neurological decline. Once neuronal destruction has occurred, even the institution of effective therapy is unlikely to improve the resultant neurological deficits. Figure 1 is a schematic representation of glioma follow-up imaging and the potential harms of early or delayed diagnosis of progression.
Figure 1.
Time to progression (TTP) and the adverse consequences of early or late diagnosis.
Response assessment in the clinic
Most patients with glioma are treated with standard of care therapies and are not on clinical trials. In this setting, what patients and clinicians care about is the presence or absence of convincing evidence of tumor growth, i.e. imaging changes that cannot be explained by technical factors or the imaging pitfalls to be discussed. Thresholds for defining progression based on percentage change are useful only to the extent that they correlate with convincing growth.
Because the convincing growth standard is inherently subjective, more technical definitions of progression are necessary in clinical trials, where it is important that a uniform definition of progression is used. The Macdonald criteria were the first widely used glioma response criteria system, and incorporated bidirectional measurement of contrast-enhancing tumor as well as information about patient clinical status and corticosteroid use.4 The four categories of response recognized by the Macdonald criteria were complete response, which requires complete disappearance of all enhancing disease; partial response, requiring ≥50% decrease in the sum of products of two perpendicular dimensions of all measurable lesions; progression, defined by a ≥ 25% increase in the sum of the products of all lesions or the development of a new lesion; and stable disease, which encompasses all other situations.
More recently, the Response Assessment in Neuro-Oncology (RANO) working group has released several brain tumor response criteria relevant to a variety of situations.5 The first RANO criteria to be published concerned high-grade gliomas and maintained the general structure of the Macdonald criteria, with four categories of response (CR, PR, SD, and PD).6 There are several differences between the RANO and Macdonald criteria, including assessment of non-enhancing disease and limits on declaring PD soon after the completion of radiation; these will be discussed in greater depth elsewhere in this review.
All of the widely used clinical trial glioma response criteria rely on changes in bidirectional measurements. The primary benefit of this approach is simplicity, allowing these criteria to be used across different institutions and imaging acquisition protocols. However, any bi-dimensional or three-plane measurement provides only an approximation of true tumor volume, and the selection of reproducible measurement planes can be difficult in irregularly shaped tumors.7 Aside from issues with measurement, standards based on percentage change impact tumors of varying sizes differently. A tumor measuring 1 cm by 1 cm at baseline only needs to grow slightly to demonstrate a 25% increase in the product of the perpendicular diameters, whereas a large tumor must grow more to meet this standard. Other potentially problematic measurements include tumors with cystic components and those abutting the enhancing walls of a resection cavity.8 In the future, volumetric measurements may prove useful in these situations, but this technique is not yet in widespread use.9
Response assessment pitfalls
Corticosteroid effect
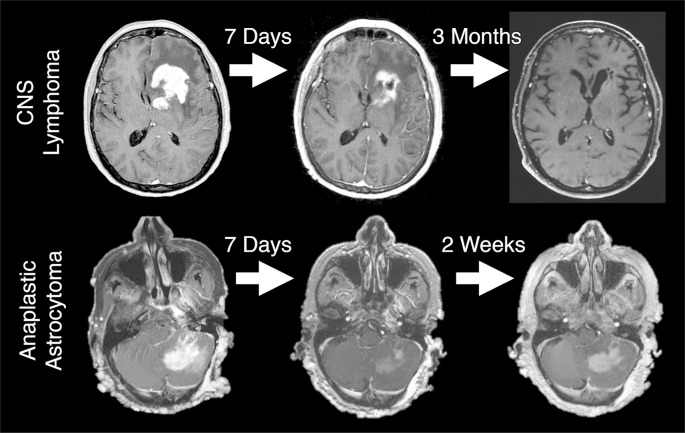
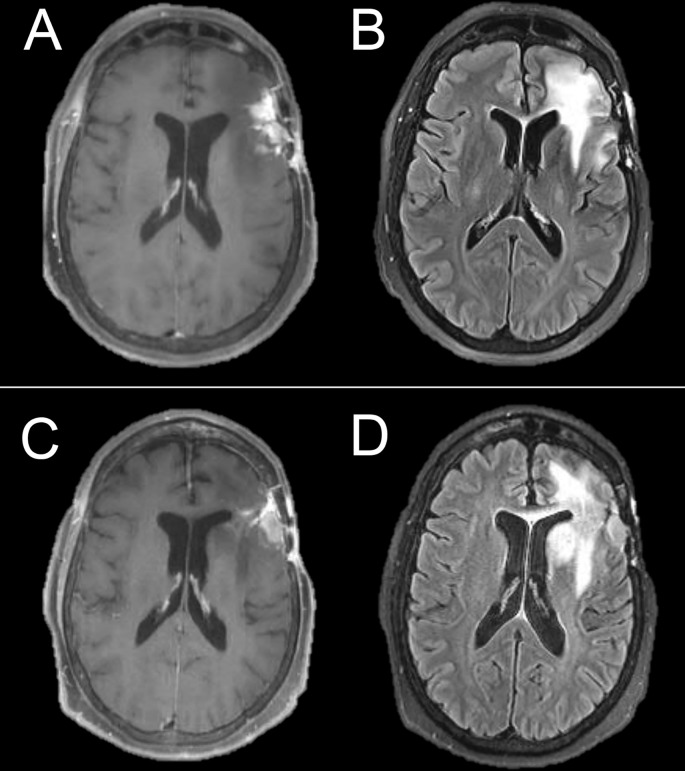
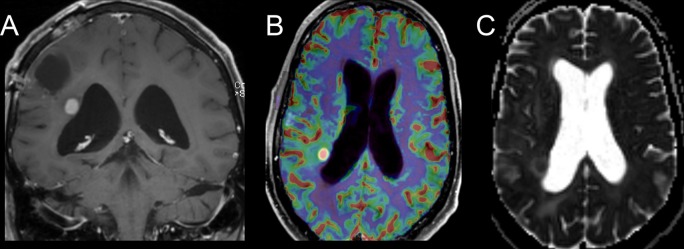
Dexamethasone has been used for decades to treat edema associated with high-grade brain tumors.10 Corticosteroids reduce vascular permeability, and can reduce both contrast enhancement and peritumoral T2/ [fluid-attenuated inversion-recovery (FLAIR)] abnormality, mimicking tumor response to therapy. Conversely, corticosteroid dose reductions in patients with high-grade gliomas may result in radiographic changes suggestive of tumor progression. Both the Macdonald and RANO response criteria incorporate information on corticosteroid dosing, but this information is not always available to radiologists at the time of MRI interpretation. Without corticosteroid dosing information it is not technically possible to assign a designation of progression or response, and a descriptive account of the changes is often more appropriate. Figure 2 shows examples of imaging change associated with corticosteroids in a patient with primary central nervous system lymphoma (PCNSL), the prototypical steroid-responsive intracranial tumor, and in a patient with anaplastic astrocytoma. This figure illustrates that substantial and potentially misleading steroid responses can be seen in high-grade glioma. Corticosteroids are used far less frequently in patients with low-grade glioma, as the T2/FLAIR abnormality in low-grade glioma primarily indicates infiltrative tumor rather than vasogenic edema.
Figure 2.
Significant response to corticosteroids in a patient with PCNSL, the prototypical steroid-responsive intracranial tumor (top), and in an anaplastic astrocytoma (bottom). In the anaplastic astrocytoma, the improvement was transient, in contrast to the prolonged effect in PCNSL. PCNSL, primary central nervous system lymphoma.
Post-surgical change
Gross total resection of tumor, provided that it can be performed with minimal morbidity, significantly improves survival in patients with infiltrating glioma.11 Gross total resection of tumor is generally defined as resection of the contrast-enhancing portion of high-grade glioma or the resection of all T2/FLAIR abnormality in non-enhancing low-grade glioma. Extensive subtotal resection is likewise beneficial, while the benefit of less substantial debulking surgery remains controversial.11–13 Following surgery, it is common to have either a thin rim or larger foci of devascularized tissue adjacent to the resection cavity, from either direct interruption of the blood supply or pressure necrosis.14 This tissue behaves radiographically just like infarcted tissue from any other cause, with early diffusion restriction, contrast enhancement in the subacute phase, and late findings of gliosis. This is important to note, since enhancement at the surgical site could represent either subacute post-surgical changes or early tumor progression in a patient post-resection who is not imaged until days to weeks after surgery. Post-surgical changes may occur regardless of tumor grade, but are more likely to present a diagnostic dilemma in patients who had contrast-enhancing tumors prior to surgery.
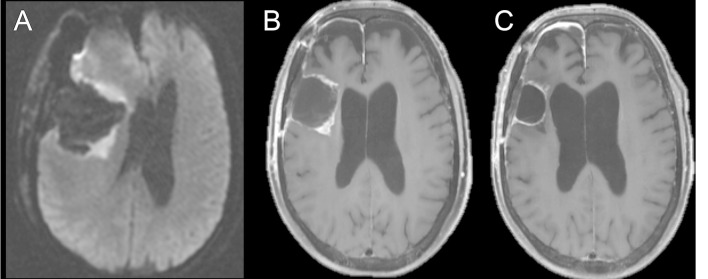
Fortunately, post-surgical change is the easiest of glioma imaging response pitfalls to avoid, by obtaining a baseline MRI soon after surgery. If an area of restricted diffusion on the post-operative MRI demonstrates contrast enhancement on the subsequent study, this suggests evolution of post-surgical changes. On the other hand, new enhancement in an area that did not demonstrate restricted diffusion is worrisome for progression. In recognition of this issue, the National Comprehensive Cancer Network recommends that a post-operative MRI should be obtained within 24 to 72 h of surgery for determination of the extent of resection.15 Figure 3 demonstrates the characteristic evolution of post-operative changes mimicking progression.
Figure 3.
Axial diffusion-weighted image of a patient with glioblastoma obtained immediately after surgical resection (A) shows restricted diffusion about the periphery of the surgical cavity. Axial T1 weighted post-contrast image (B) obtained 3 weeks after surgery for radiation planning demonstrates contrast enhancement in the areas of previous restricted diffusion, consistent with subacute enhancing infarction, which would otherwise be ambiguous without the previous DWI. Follow-up axial T1 weighted post-contrast image (C) demonstrates reduced contrast enhancement, consistent with evolution of post-surgical changes. DWI, diffusion-weighted imaging.
Chemoradiotherapy-related change
Chemoradiotherapy-related changes are the prototypical, and still most common, imaging pitfall encountered in glioma response assessment imaging. The category of CRC includes pseudoprogression and radiation necrosis, terms that are used variably, and sometimes interchangeably, in the glioma literature. Most authors do distinguish between these entities, with a major difference being onset during or shortly after therapy for pseudoprogression, and a greater delay for radiation necrosis.16
The term pseudoprogression generally refers to asymptomatic new or increased contrast enhancement that develops during or soon after chemoradiotherapy and then improves or resolves without further treatment (Figure 4).17 While pseudoprogression can occur after radiotherapy alone, it was first widely recognized after the adoption of concurrent radiation and oral temozolomide chemotherapy as the standard-of-care treatment for newly diagnosed glioblastoma in the mid-2000s.18 In classic descriptions, pseudoprogression is evident on the first post-chemoradiotherapy MRI, usually obtained approximately 1 month after the end of radiation, then progressively improves or resolves thereafter. In reality the situation is more complicated. Onset of pseudoprogression may be delayed for several months, and the imaging findings may increase on serial scans before ultimately plateauing or resolving. Reported rates of pseudoprogression vary widely in the literature, from less than 10% to greater than 30%, due to factors including heterogeneous patient populations, different imaging protocols, and divergent gold standard criteria.19 A recent meta-analysis of 73 high-grade glioma studies totaling 2603 patients found that 36% of patients demonstrated pseudoprogression (95% confidence interval 33–40%).20 Patients who experience pseudoprogression have been suggested to have better long-term survival than patients that do not, even after controlling for other important prognostic factors, which is intuitive presuming the radiographic findings of pseudoprogression reflect tumor response to therapy.21 This concept is likely generalizable to pseudoprogression associated with immunotherapy, to be discussed later.
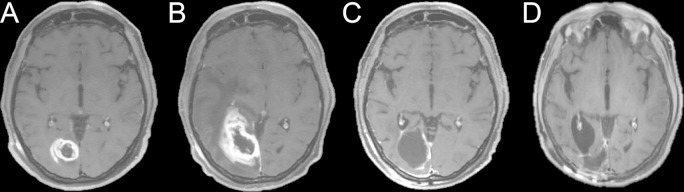
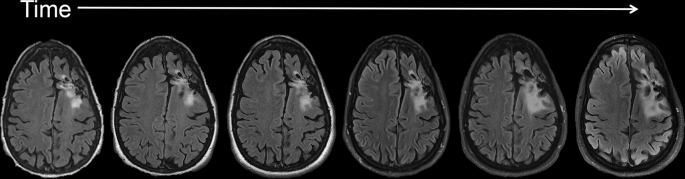
Figure 4.
Axial T1 weighted post-contrast images of a patient with glioblastoma (A-D). Pre-chemoradiotherapy (A). Initial post-chemoradiotherapy (B) images demonstrate significantly increased enhancement at the tumor site. Due to associated symptoms, the patient underwent resection of this region. Pathology showed primarily radiation effect. Post-operative MR (C) demonstrates no significant residual disease, confirming the previous findings represented pseudoprogression. 2-year follow-up imaging (D) demonstrates stability despite lack of additional interval treatment.
Other well-described late radiation sequelae include a delayed vasculopathy, resulting in cerebral infarction, and vascular proliferative lesions, such as capillary telangiactasias and cavernous malformations. These conditions tend to occur years after radiation and are thus seen more frequently in patients with lower grade tumors who have longer life expectancies. A spectrum of acute, late-onset neurological manifestations of brain irradiation, consisting of potentially reversible focal neurological deficits, seizures, and imaging abnormalities have been reported; however, the rarity of these conditions puts them beyond the scope of this review.22
The RANO working group proposed a definition of pseudoprogression for the purpose of identifying tumor progression in clinical trials. Per RANO, in the setting of increased contrast enhancement at MRI within 12 weeks after the completion of chemoradiotherapy, progression can only be defined if the enhancement occurs outside of the high-dose radiation field or if there is unequivocal evidence of viable tumor on histopathological sampling.6 These rules apply to any new enhancement within the high-dose field, whether it is expansion of previous enhancement or a new focus of enhancement within a previously non-enhancing tumor. The essence of this definition can also be applied to patients treated outside of clinical trials, although in this case there is more room for individual judgement. While the RANO criteria effectively prevent the premature diagnosis of classical pseudoprogression as true progression, they are of limited utility for late pseudoprogression and radiation necrosis.
The vast majority of the literature regarding pseudoprogression concerns patients with glioblastoma, but World Health Organization grade II and III gliomas can also demonstrate pseudoprogression.23,24 Knowledge of patient tumor type and the expected behavior of that tumor is thus essential in predicting progression vs pseudoprogression. For example, oligodendroglioma [by definition isocitrate dehydrogenase (IDH)-mutant and 1p/19q co-deleted] is a treatment-responsive tumor with a much better prognosis than glioblastoma. Given the aggressive nature of glioblastoma, a patient may experience true tumor progression during or within months after completion of first-line therapy, while this would be distinctly unusual in oligodendroglioma. Similarly, in patients with glioblastoma, several reports have demonstrated that pseudoprogression occurs more commonly in methylguanine-DNA methyltransferase methylated tumors and more often in IDH-mutant tumors than in IDH wild-type tumors.25,26
Pseudoresponse to antiangiogenic therapy
Prior to the introduction of antiangiogenic therapies for high-grade glioma, most notably bevacizumab (Avastin TM), radiographic response to treatment of infiltrating glioma was infrequently seen. Effective therapies were instead defined by their ability to induce a prolonged cessation of tumor growth. On the other hand, initiation of antiangiogenic therapy regularly results in significant radiographic improvements in both contrast enhancement and T2/FLAIR signal surrounding the enhancing core of a high-grade glioma.27 However, patients receiving antiangiogenic therapy may demonstrate discordant responses, wherein the degree of enhancement diminishes even as the bulk of T2/FLAIR hyperintense tumor increases, a situation termed “pseudoresponse” (Figure 5).
Figure 5.
T1weighted post-contrast (A and C) and FLAIR (B and D) MR images obtained before (A–B) and after (C–D) bevacizumab therapy. The degree of contrast enhancement is mildly reduced after treatment, but the extent of infiltrative mass-like FLAIR abnormality has increased, particularly in the left basal ganglia. This suggests non-enhancing tumor progression. FLAIR, fluid-attenuated inversion-recovery.
The term pseudoresponse was coined to indicate discordance between imaging and tumor behavior, analogous to the well-recognized phenomenon of pseudoprogression.28 However, the situation of response to antiangiogenic agents is more complex. The “response” component of pseudoresponse typically occurs early, with maximal imaging improvement generally apparent on the first post-treatment imaging. Evidence of non-enhancing progression, on the other hand, can be delayed by months. Early on, the increase in non-enhancing tumor bulk may be masked by a simultaneous decrease in peritumoral vasogenic edema; subtle progression later may be mistaken for technical scan-to-scan variability or evolving radiation-related changes. By the time mass-like T2/FLAIR progression is unambiguously present, antiangiogenic therapy may have been ongoing for weeks or months. As such, descriptive terms such as non-enhancing progression or infiltrative tumor growth may be preferable to pseudoresponse.
The RANO criteria contains language addressing this non-enhancing growth in patients on antiangiogenic therapies, noting that along with definitions of progression based on enhancing disease, progression may also be defined as a “significant increase” in T2/FLAIR disease burden that cannot be attributed to changes in corticosteroid dosing or comorbid events.6 Recently, the importance of early recognition of non-enhancing tumor growth has been questioned, given the subjective nature of identifying a “significant increase”, similar performance of RANO and Macdonald criteria for prediction of overall survival, and lack of proven effective treatment options after bevacizumab failure.29,30 At present, however, progression of non-enhancing disease is considered clinically important progression, and radiologists must carefully assess the T2/FLAIR tumor burden in any patient on antiangiogenic therapy. Several recent randomized clinical trials failed to demonstrate any survival benefit associated with bevacizumab therapy in patients with newly-diagnosed or recurrent glioblastoma, and it is possible that decreasing bevacizumab use in the future will eventually make the phenomenon of glioblastoma pseudoresponse one of primarily historical interest. Of note, bevacizumab is rarely used to treat low-grade glioma.
Indolent progression
Low-grade infiltrating gliomas carry a much better prognosis than high-grade tumors, but nonetheless demonstrate inevitable progression.31 A common error, facilitated by many radiology imaging review systems, is to evaluate the current imaging study along with only one or two of the most recent studies for comparison. While this approach may be sufficient in high-grade tumors, it is not adequate in slowly growing tumors. In these cases, additional comparison should be made to older images, ideally with reference to the first MRI study obtained after the most recent therapeutic intervention. Although this pitfall is well-known, “missed” progression still occurs in clinical practice and may only be recognized in retrospect after a patient develops new symptoms or new contrast enhancement (Figure 6). A potential solution to this issue, beyond increased vigilance on the part of the interpreting radiologist, is to design imaging systems that automatically retrieve relevant older studies for comparison.
Figure 6.
Serial T2 weighted FLAIR MR images (some interval scans omitted) over several years, demonstrating a case of indolent progression in a low-grade astrocytoma. The change between any two scans within this time sequence is minimal, demonstrating the need for comparison to more remote imaging studies in order to detect slow and subtle change over time. FLAIR, fluid-attenuated inversion-recovery.
Alternating electric field therapy
Alternating electric field therapy, also known as tumor treating fields, represents a relatively new and novel treatment for high-grade infiltrating gliomas.32 Currently, the only commercially available system is the Optune™ device. This FDA approved device for newly diagnosed and recurrent glioblastoma consists of electrode arrays connected to a battery pack. A patient treated with alternating electric field therapy wears the scalp electrode arrays for 18 or more hours each day. The proposed mechanism of action is impairment of microtubule function and subsequent disruption of cellular mitosis. As the electrode array is removed prior to MR imaging, treatment with alternating electric fields will be apparent only through a review of the clinical record. This is important, as tumor growth within the first 4 weeks after initiation of therapy does not necessarily predict long-term treatment failure.33 As such, it may be useful to obtain an updated “baseline” MR after a month of treatment.
Immunomodulatory therapy
Over the last decade, immunotherapy approaches to cancer treatment have been successfully translated into practice, with proven efficacy in tumors such as melanoma, lung cancer, prostate cancer, and renal cell carcinoma. The category of immunomodulatory therapy includes treatments that work by a variety of mechanisms, including tumor vaccines, immune checkpoint inhibitors, and modified T cells.34 While no immunotherapy regimen has yet received FDA approval for use in patients with infiltrating glioma, there are numerous ongoing clinical trials, and immunotherapies approved for other tumors may be used on an off-label basis in patients with glioblastoma. Currently, immunotherapy clinical trials and off-label use almost exclusively involve patients with high-grade tumors.
As immunotherapy induces a tumor-specific immune response, successful treatment often results in inflammatory changes characterized by increased contrast enhancement and edema.35 In contrast to the relatively predictable temporal course of radiochemotherapy-related pseudoprogression, the pseudoprogression equivalent associated with immunotherapy may be more delayed from the onset of therapy and may progress for longer periods of time, presenting significant difficulty for specific differentiation of treatment effect from tumor growth (Figure 7).
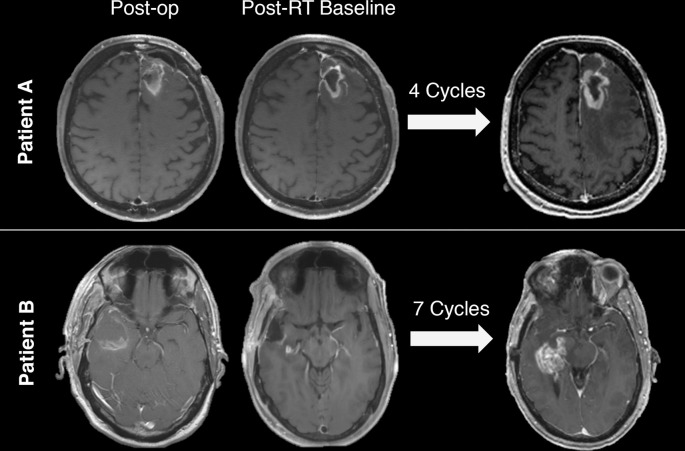
Figure 7.
Two patients with glioblastoma treated with immunotherapy who demonstrated increased enhancement on axial T1 weighted post-gadolinium images after multiple cycles of immunotherapy. Both patients went on to surgery; pathology of Patient A demonstrated treatment effect while Patient B had recurrent tumor.
The RANO working group released recommendations titled Immunotherapy Response Assessment in Neuro-Oncology to help standardize how this issue is resolved in the context of clinical trials.36 Per Immunotherapy Response Assessment in Neuro-Oncology, if radiographic progression unaccompanied by a significant clinical decline occurs within the first 6 months of an immunotherapy regimen, treatment should be continued and the patient should be re-imaged 3 months later unless there is clinical decline. If further radiographic evidence of progression is noted at 3 months, then date of progression is backdated to the time of the original MR scan.
Imaging tools
Standard anatomic MR imaging is the technique of choice for glioma follow-up, and neither the Macdonald nor the RANO criteria for response assessment directly incorporate imaging techniques beyond post-contrast T1 weighted imaging (both Macdonald and RANO) and T2/FLAIR imaging (RANO only). Nonetheless, most MR protocols also include diffusion-weighted imaging (DWI), and many centers routinely obtain perfusion imaging and/or MR spectroscopy in patients with brain tumors. Positron emission tomography (PET) imaging is also advancing rapidly, although it remains less frequently obtained than MR in patients with brain tumors. Each of these techniques offers the possibility of interrogating tumor physiology rather than simply anatomy, and it is likely that physiological imaging will continue to play an increasing role in glioma response assessment.
Advanced MR techniques
Perfusion MR can be used to quantify cerebral blood flow. Generally speaking, cerebral blood volume (CBV) is expected to be higher in viable glioma tissue than in radiation necrosis, although the situation is less clear in pseudoprogression.37–44 In practice, there is frequently overlap between CBV values in viable glioblastoma and radiation-induced changes, partly because treated tumor tends to contain both viable tumor cells and radiation effect rather than either in isolation. Specificity may be improved by techniques such as histogram and voxel-wise analyses of perfusion data, but these are post-processing intensive.45,46 Longitudinal quantitative analysis of perfusion changes within a lesion can be useful in characterizing tumor stability or progression, provided a sufficiently reproducible perfusion technique is used.47 Most glioma perfusion imaging is obtained with dynamic susceptibility contrast technique. Nevertheless, the more technically demanding dynamic contrast-enhanced perfusion imaging method allows measurement of volume transfer coefficient ktrans and initial area under the curve, both of which may be useful in differentiating recurrent glioblastoma from treatment-related changes.43,48–50 Figure 8 shows an example of evolving pseudoprogression with corresponding CBV perfusion images. While a valuable method, perfusion MR has a number of potential drawbacks.51 First, selection of the region of interest for analysis is operator-dependent, and region of interest-based methods may yield different results than histogram analysis.52 Secondly, the technical parameters of image acquisition such as the use of pre-load dosing can impact the results.53 Finally, different perfusion analysis software packages may produce significantly different results, even when processing identical raw MR perfusion data.54
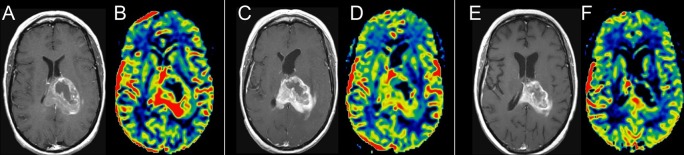
Figure 8.
Axial T1 weighted post-contrast and axial MR perfusion rCBV image pairs from time points prior to chemoradiotherapy (A & B), 1 month after the end of chemoradiotherapy (C & D), and 3 months after the end of chemoradiotherapy (E & F). The initial post-treatment images show increased enhancement but reduced rCBV, suggestive of pseudoprogression. This is confirmed by decreased enhancement and continued decreased rCBV on the subsequent study. rCBV, relative cerebral blood volume.
DWI, and in particular apparent diffusion coefficient (ADC) imaging, can offer insight into glioma response assessment. High-grade gliomas grow with increased cell density relative to normal brain, manifesting as restricted diffusion and low ADC values.55
Advanced analysis techniques, such as histogram analysis and functional diffusion maps,56–61 may improve the ability of ADC analysis to differentiate recurrent tumor from treatment-related changes (Figure 9).55 Diffusion and ADC analysis results may be confounded by the complex relationship between tumor heterogeneity and diffusion characteristics, as edema and necrosis can increase ADC values, while recently infarcted tissue (e.g. in the post-surgical setting or due to vascular occlusion) demonstrates markedly reduced ADC values. Further, various b-value levels for ADC analysis have been investigated, and there is not currently a consensus on the optimal technique.62
Figure 9.
Coronal T1 weighted post-contrast image (A) of a patient with glioblastoma demonstrates a focus of enhancement along the right lateral ventricle, deep to the resection cavity, which was new since the most recent comparison imaging. Perfusion MR image (B) demonstrates increased rCBV and the ADC map (C) shows restricted diffusion, both consistent with progressive, cellular, vascular tumor. ADC, apparent diffusion coefficient; rCBV, relative cerebral blood volume.
MR spectroscopy (MRS) allows for the non-invasive detection and quantification of metabolites within tumor tissue and normal brain. Commonly assessed metabolites include choline, creatine, N-acetylaspartate (NAA), and lactate. The relative ratios of these differ in normal brain, tumor, and areas of radiation effect. In gliomas, increasing tumor grade correlates with increasing lactate and lipids and decreasing NAA and creatine.63 Choline-NAA and choline-creatine ratios thus increase with tumor grade, and the ratios may be more useful than absolute metabolite concentrations. Radiation necrosis characteristically demonstrates reduced choline-creatine and choline-NAA ratios relative to recurrent tumor, as well as an elevated lipid-lactate peak.64 While a meta-analysis of glioma MRS studies reported excellent sensitivity and specificity for the technique,65 use of and enthusiasm for MRS varies widely in practice, and it is very rarely employed in patients with brain tumors at the author’s institution. A practical limitation of MRS for the question of progression vs pseudoprogression is that almost all patients who have recently received chemoradiotherapy have a mixture of treatment effect and viable tumor at pathology.
Gliomas harboring mutations in the IDH gene, including all oligodendrogliomas, most astrocytomas, and a minority of glioblastomas, contain elevated levels of 2-hydroxyglutarate (2HG) relative to gliomas with non-mutated IDH.66 MRS can be used to assess 2HG levels, although the technique is challenging as the size of the 2HG MRS peak is quite small relative to the more commonly assessed metabolites.67,68 Longitudinal quantification of 2HG concentrations may prove useful in tumor response assessment. For example, decreasing levels have been noted after treatment whereas rising levels may precede other MR evidence of tumor progression.69 While this technique remains largely investigational, it has been incorporated into clinical practice in some centers.70
Positron Emission Tomography
The majority of PET imaging obtained in patients with cancer utilizes 18F-Fluorodeoxyglucose (FDG), allowing for the differentiation of hypermetabolic tumor from lesser metabolism in normal tissues. While this approach has been explored in patients with brain tumors, high levels of FDG metabolism in normal brain result in relatively unfavorable tumor-to-background ratios. Thus, the limited specificity of this technique has precluded its use in routine clinical practice.71,72
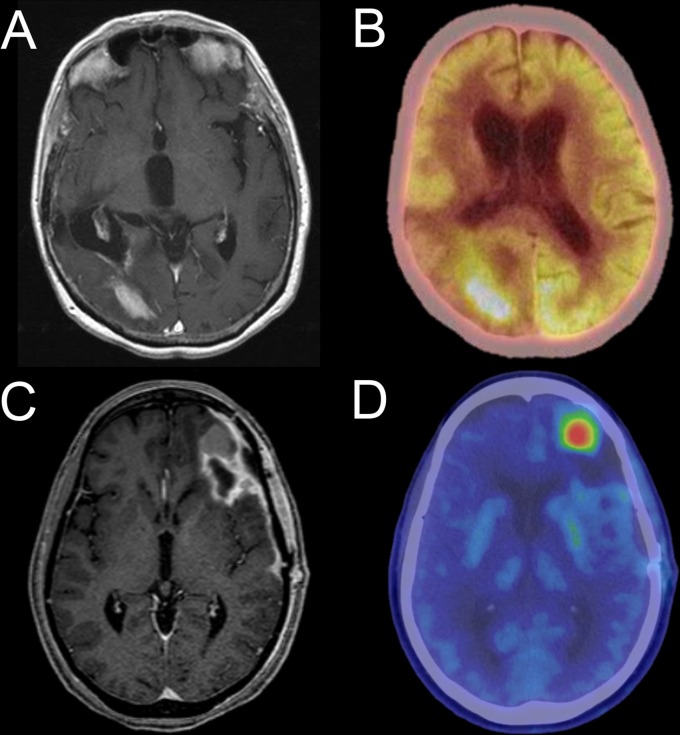
The three most frequently used non-FDG PET agents in glioma are the amino acid agents O-(2-18 F-fluoroethyl)-l-tyrosine (FET), 3,4-dihydroxy-6-18 F-fluoro-l-phenylalanine (FDOPA), and11 C-methionine (MET). FET, FDOPA, and MET are all transported into cells by the LAT1/2 L-type amino acid transporter system, which is upregulated in glioma. These agents have demonstrated utility in surgical/radiation planning as well as response assessment.73 While the specificity of amino acid PET for progression vs pseudoprogression is superior to FDG PET, it is not perfect and uptake in inflammatory tissue remains a potential confounder.74 Currently, none of these amino acid PET tracers is FDA approved for use in the US, although they are clinically available elsewhere and their use in multiple clinical settings is recommended by the European Association for Neuro-Oncology.75 Figure 10 demonstrates examples of FDG and FDOPA PET imaging in patients with recurrent glioma.
Figure 10.
Axial T1 weighted post-contrast MR (A) and FDG PET/CT images of a patient with a right parieto-occipital GBM demonstrate tumor hypermetabolism on a background of normal, relatively high cerebral metabolism. Axial T1 weighted post-contrast MR (C) and FDOPA PET/CT (D) images of a different patient with GBM show focal FDOPA uptake in a contrast-enhancing nodule along the anterior border of the left frontal tumor resection cavity, indicative of recurrent disease. Of note, different PET display color palettes are used for the example FDG and FDOPA studies. FDG, 18F-Fluorodeoxyglucose; FDOPA, 3,4-dihydroxy-6-18 F-fluoro-l-phenylalanine; GBM, glioblastoma multiforme.
Numerous additional PET agents have potential utility in glioma imaging. The amino acid PET agent18 F-fluciclovine (FACBC), which is FDA approved for prostate cancer imaging, primarily utilizes the glutamine transporter ASCT2 rather than the previously discussed LAT1/2 transport system and has shown early promise in glioma imaging.76 Various PET agents targeting prostate specific membrane antigen have been shown to bind to tumor blood vessels, including those in glioma, and at least one of these agents is likely to gain FDA approval in the foreseeable future.77 A wide variety of other PET agents have been described, allowing imaging of DNA replication, neoangiogenesis, and hypoxia, amongst other targets.74
Machine learning
The topics of artificial intelligence and machine learning have recently generated a great deal of interest in radiology generally and brain tumor imaging in specific.78 While much of the current literature regarding machine learning in patients with glioma has concerned radiogenomics or tumor segmentation, several publications have examined it as a tool for differentiation of tumor progression vs pseudoprogression.79,80 While this is a rapidly advancing area of research, further replication and validation of the results will be necessary before machine learning approaches can be incorporated into routine clinical practice.
Conclusion
Neuroradiology plays an important role in the management of patients with glioma. Imaging changes may be the first sign of tumor growth, and patients can be harmed by either delayed or erroneous identification of progression. Familiarity with common, predictable imaging pitfalls can directly affect treatment decisions. Clear communication of the radiographic impression to the treating clinician, with analysis of specific differences from prior imaging in relation to the clinical context, is imperative. A thorough review of the patient medical record and a detailed understanding of the imaging implications of common tumor treatments can help clinicians and radiologists avoid many of the pitfalls in glioma response assessment. In the longer term, volumetric analysis and advanced MR and PET imaging techniques may allow more precise and specific identification of tumor progression.
Footnotes
Acknowledgment: The authors acknowledge the assistance of Sonia Watson, PhD and Desiree Lanzino, PT, PhD, in editing the manuscript.
Competing interests: A preliminary form of this manuscript was presented at the RNSA 2017 Annual Meeting as an educational exhibit (Space #: NR111-ED-X) where it received Magna Cum Laude award recognition.
Contributor Information
Derek R. Johnson, Email: johnson.derek1@mayo.edu.
Julie B. Guerin, Email: guerin.julie@mayo.edu.
Michael W. Ruff, Email: ruff.michael@mayo.edu.
Shanna Fang, Email: shannafang@gmail.com.
Christopher H. Hunt, Email: hunt.christopher@mayo.edu.
Jonathan M. Morris, Email: morris.jonathan@mayo.edu.
P. Pearse Morris, Email: morris.ppearse@mayo.edu.
Timothy J. Kaufmann, Email: kaufmann.timothy@mayo.edu.
REFERENCES
- 1.Buckner JC, Shaw EG, Pugh SL, Chakravarti A, Gilbert MR, Barger GR, et al. . Radiation plus procarbazine, CCNU, and vincristine in low-grade glioma. N Engl J Med Overseas Ed 2016; 374: 1344–55. doi: 10.1056/NEJMoa1500925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson DR, O’Neill BP. Glioblastoma survival in the united States before and during the temozolomide era. J Neurooncol 2012; 107: 359–64. doi: 10.1007/s11060-011-0749-4 [DOI] [PubMed] [Google Scholar]
- 3.Nayak L, DeAngelis LM, Brandes AA, Peereboom DM, Galanis E, Lin NU, et al. . The neurologic assessment in neuro-oncology (NANO) scale: a tool to assess neurologic function for integration into the response assessment in neuro-oncology (RANO) criteria. Neuro Oncol 2017; 19: 625–35. doi: 10.1093/neuonc/nox029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Macdonald DR, Cascino TL, Schold SC, Cairncross JG, Jr. CJG. Response criteria for phase II studies of supratentorial malignant glioma. Journal of Clinical Oncology 1990; 8: 1277–80. doi: 10.1200/JCO.1990.8.7.1277 [DOI] [PubMed] [Google Scholar]
- 5.Eisele SC, Wen PY, Lee EQ. Assessment of brain tumor response: RANO and its offspring. Curr Treat Options Oncol 2016; 17: 35. doi: 10.1007/s11864-016-0413-5 [DOI] [PubMed] [Google Scholar]
- 6.Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, et al. . Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. Journal of Clinical Oncology 2010; 28: 1963–72. doi: 10.1200/JCO.2009.26.3541 [DOI] [PubMed] [Google Scholar]
- 7.Provenzale JM, Ison C, Delong D. Bidimensional measurements in brain tumors: assessment of interobserver variability. AJR Am J Roentgenol 2009; 193: W515–W522. doi: 10.2214/AJR.09.2615 [DOI] [PubMed] [Google Scholar]
- 8.Kanaly CW, Ding D, Mehta AI, Waller AF, Crocker I, Desjardins A, et al. . A novel method for volumetric MRI response assessment of enhancing brain tumors. PLoS One 2011; 6: e16031. doi: 10.1371/journal.pone.0016031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blomstergren A, Rydelius A, Abul-Kasim K, Lätt J, Sundgren PC, Bengzon J. Evaluation of reproducibility in MRI quantitative volumetric assessment and its role in the prediction of overall survival and progression-free survival in glioblastoma. Acta Radiol 2018;: 284185118786060. doi: 10.1177/0284185118786060 [DOI] [PubMed] [Google Scholar]
- 10.Galicich JH, French LA, Melby JC. Use of dexamethasone in treatment of cerebral edema associated with brain tumors. J Lancet 1961; 81: 46–53. [PubMed] [Google Scholar]
- 11.Trifiletti DM, Alonso C, Grover S, Fadul CE, Sheehan JP, Showalter TN. Prognostic implications of extent of resection in glioblastoma: Analysis from a large database. World Neurosurg 2017; 103: 330–40. doi: 10.1016/j.wneu.2017.04.035 [DOI] [PubMed] [Google Scholar]
- 12.Chaichana KL, Jusue-Torres I, Navarro-Ramirez R, Raza SM, Pascual-Gallego M, Ibrahim A, et al. . Establishing percent resection and residual volume thresholds affecting survival and recurrence for patients with newly diagnosed intracranial glioblastoma. Neuro Oncol 2014; 16: 113–22. doi: 10.1093/neuonc/not137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grabowski MM, Recinos PF, Nowacki AS, Schroeder JL, Angelov L, Barnett GH, et al. . Residual tumor volume versus extent of resection: predictors of survival after surgery for glioblastoma. J Neurosurg 2014; 121: 1115–23. doi: 10.3171/2014.7.JNS132449 [DOI] [PubMed] [Google Scholar]
- 14.Smith JS, Cha S, Mayo MC, McDermott MW, Parsa AT, Chang SM, et al. . Serial diffusion-weighted magnetic resonance imaging in cases of glioma: distinguishing tumor recurrence from postresection injury. J Neurosurg 2005; 103: 428–38. doi: 10.3171/jns.2005.103.3.0428 [DOI] [PubMed] [Google Scholar]
- 15.Nabors LB, Portnow J, Ammirati M, Baehring J, Brem H, Butowski N, et al. . NCCN Guidelines Insights: central nervous system cancers, version 1.2017. J Natl Compr Canc Netw 2017; 15: 1331–45. doi: 10.6004/jnccn.2017.0166 [DOI] [PubMed] [Google Scholar]
- 16.Sanghera P, Rampling R, Haylock B, Jefferies S, McBain C, Rees JH, et al. . The concepts, diagnosis and management of early imaging changes after therapy for glioblastomas. Clin Oncol 2012; 24: 216–27. doi: 10.1016/j.clon.2011.06.004 [DOI] [PubMed] [Google Scholar]
- 17.Brandsma D, Stalpers L, Taal W, Sminia P, van den Bent MJ. Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol 2008; 9: 453–61. doi: 10.1016/S1470-2045(08)70125-6 [DOI] [PubMed] [Google Scholar]
- 18.Chaskis C, Neyns B, Michotte A, De Ridder M, Everaert H. Pseudoprogression after radiotherapy with concurrent temozolomide for high-grade glioma: clinical observations and working recommendations. Surg Neurol 2009; 72: 423–8. doi: 10.1016/j.surneu.2008.09.023 [DOI] [PubMed] [Google Scholar]
- 19.Kruser TJ, Mehta MP, Robins HI. Pseudoprogression after glioma therapy: a comprehensive review. Expert Rev Neurother 2013; 13: 389–403. doi: 10.1586/ern.13.7 [DOI] [PubMed] [Google Scholar]
- 20.Abbasi AW, Westerlaan HE, Holtman GA, Aden KM, van Laar PJ, van der Hoorn A. Incidence of tumour progression and pseudoprogression in high-grade gliomas: a systematic review and meta-analysis. Clin Neuroradiol 2018; 28: 401–11. doi: 10.1007/s00062-017-0584-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brandes AA, Franceschi E, Tosoni A, Blatt V, Pession A, Tallini G, et al. . MGMT promoter methylation status can predict the incidence and outcome of pseudoprogression after concomitant radiochemotherapy in newly diagnosed glioblastoma patients. Journal of Clinical Oncology 2008; 26: 2192–7. doi: 10.1200/JCO.2007.14.8163 [DOI] [PubMed] [Google Scholar]
- 22.Black DF, Bartleson JD, Bell ML, Lachance DH. SMART: stroke-like migraine attacks after radiation therapy. Cephalalgia 2006; 26: 1137–42. doi: 10.1111/j.1468-2982.2006.01184.x [DOI] [PubMed] [Google Scholar]
- 23.Bronk JK, Guha-Thakurta N, Allen PK, Mahajan A, Grosshans DR, McGovern SL. Analysis of pseudoprogression after proton or photon therapy of 99 patients with low grade and anaplastic glioma. Clin Transl Radiat Oncol 2018; 9: 30–4. doi: 10.1016/j.ctro.2018.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hernandez-Lain A, Hilario A, Sepulveda JM, Cantero D, Ramos A, Perez-Nuñez A. Temozolomide induces radiologic pseudoprogression and tumor cell vanishing in oligodendroglioma. Neurology 2016; 87: 114–5. doi: 10.1212/WNL.0000000000002810 [DOI] [PubMed] [Google Scholar]
- 25.Li H, Li J, Cheng G, Zhang J, Li X. IDH mutation and MGMT promoter methylation are associated with the pseudoprogression and improved prognosis of glioblastoma multiforme patients who have undergone concurrent and adjuvant temozolomide-based chemoradiotherapy. Clin Neurol Neurosurg 2016; 151: 31–6. doi: 10.1016/j.clineuro.2016.10.004 [DOI] [PubMed] [Google Scholar]
- 26.Motegi H, Kamoshima Y, Terasaka S, Kobayashi H, Yamaguchi S, Tanino M, et al. . IDH1 mutation as a potential novel biomarker for distinguishing pseudoprogression from true progression in patients with glioblastoma treated with temozolomide and radiotherapy. Brain Tumor Pathol 2013; 30: 67–72. doi: 10.1007/s10014-012-0109-x [DOI] [PubMed] [Google Scholar]
- 27.Vredenburgh JJ, Desjardins A, Herndon JE, Marcello J, Reardon DA, Quinn JA, et al. . Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. Journal of Clinical Oncology 2007; 25: 4722–9. doi: 10.1200/JCO.2007.12.2440 [DOI] [PubMed] [Google Scholar]
- 28.Clarke JL, Chang S. Pseudoprogression and pseudoresponse: hallenges in brain tumor imaging. Curr Neurol Neurosci Rep 2009; 9: 241–6. doi: 10.1007/s11910-009-0035-4 [DOI] [PubMed] [Google Scholar]
- 29.Ellingson BM, Wen PY, Cloughesy TF. Modified criteria for radiographic response assessment in glioblastoma clinical trials. Neurotherapeutics 2017; 14: 307–20. doi: 10.1007/s13311-016-0507-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang RY, Rahman R, Ballman KV, Felten SJ, Anderson SK, Ellingson BM, et al. . The impact of T2/FLAIR evaluation per RANO criteria on response assessment of recurrent glioblastoma patients treated with bevacizumab. Clinical Cancer Research 2016; 22: 575–81. doi: 10.1158/1078-0432.CCR-14-3040 [DOI] [PubMed] [Google Scholar]
- 31.van den Bent MJ, Wefel JS, Schiff D, Taphoorn MJB, Jaeckle K, Junck L, et al. . Response assessment in neuro-oncology (a report of the RANO group): assessment of outcome in trials of diffuse low-grade gliomas. Lancet Oncol 2011; 12: 583–93. doi: 10.1016/S1470-2045(11)70057-2 [DOI] [PubMed] [Google Scholar]
- 32.Stupp R, Taillibert S, Kanner A, Read W, Steinberg DM, Lhermitte B, et al. . Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma. JAMA 2017; 318: 2306–16. doi: 10.1001/jama.2017.18718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vymazal J, Wong ET. Response patterns of recurrent glioblastomas treated with tumor-treating fields. Semin Oncol 2014; 41(Suppl 6): S14–S24. doi: 10.1053/j.seminoncol.2014.09.009 [DOI] [PubMed] [Google Scholar]
- 34.Lim M, Xia Y, Bettegowda C, Weller M. Current state of immunotherapy for glioblastoma. Nat Rev Clin Oncol 2018; 15: 422–42. doi: 10.1038/s41571-018-0003-5 [DOI] [PubMed] [Google Scholar]
- 35.Aquino D, Gioppo A, Finocchiaro G, Bruzzone MG, Cuccarini V. MRI in glioma immunotherapy: Evidence, pitfalls, and perspectives. J Immunol Res 2017; 2017: 1–16. doi: 10.1155/2017/5813951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Okada H, Weller M, Huang R, Finocchiaro G, Gilbert MR, Wick W, et al. . Immunotherapy response assessment in neuro-oncology: a report of the RANO working group. Lancet Oncol 2015; 16: e534–e542. doi: 10.1016/S1470-2045(15)00088-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barajas RF, Chang JS, Segal MR, Parsa AT, McDermott MW, Berger MS, et al. . Differentiation of recurrent glioblastoma multiforme from radiation necrosis after external beam radiation therapy with dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. Radiology 2009; 253: 486–96. doi: 10.1148/radiol.2532090007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim YH, Oh SW, Lim YJ, Park C-K, Lee S-H, Kang KW, et al. . Differentiating radiation necrosis from tumor recurrence in high-grade gliomas: Assessing the efficacy of 18F-FDG PET, 11C-methionine PET and perfusion MRI. Clin Neurol Neurosurg 2010; 112: 758–65. doi: 10.1016/j.clineuro.2010.06.005 [DOI] [PubMed] [Google Scholar]
- 39.Sugahara T, Korogi Y, Tomiguchi S, Shigematsu Y, Ikushima I, Kira T, et al. . Posttherapeutic intraaxial brain tumor: the value of perfusion-sensitive contrast-enhanced MR imaging for differentiating tumor recurrence from nonneoplastic contrast-enhancing tissue. AJNR Am J Neuroradiol 2000; 21: 901–9. [PMC free article] [PubMed] [Google Scholar]
- 40.LS H, Baxter LC, Smith KA, Feuerstein BG, Karis JP, Eschbacher JM, et al. . Relative cerebral blood volume values to differentiate high-grade glioma recurrence from posttreatment radiation effect: direct correlation between image-guided tissue histopathology and localized dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging measurements. AJNR Am J Neuroradiol 2009; 30: 552–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mangla R, Singh G, Ziegelitz D, Milano MT, Korones DN, Zhong J, et al. . Changes in relative cerebral blood volume 1 month after radiation-temozolomide therapy can help predict overall survival in patients with glioblastoma. Radiology 2010; 256: 575–84. doi: 10.1148/radiol.10091440 [DOI] [PubMed] [Google Scholar]
- 42.Gahramanov S, Raslan AM, Muldoon LL, Hamilton BE, Rooney WD, Varallyay CG, et al. . Potential for differentiation of pseudoprogression from true tumor progression with dynamic susceptibility-weighted contrast-enhanced magnetic resonance imaging using ferumoxytol vs. gadoteridol: a pilot study. Int J Radiat Oncol Biol Phys 2011; 79: 514–23. doi: 10.1016/j.ijrobp.2009.10.072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim HS, Goh MJ, Kim N, Choi CG, Kim SJ, Kim JH. Which combination of MR imaging modalities is best for predicting recurrent glioblastoma? Study of diagnostic accuracy and reproducibility. Radiology 2014; 273: 831–43. doi: 10.1148/radiol.14132868 [DOI] [PubMed] [Google Scholar]
- 44.Seeger A, Braun C, Skardelly M, Paulsen F, Schittenhelm J, Ernemann U, et al. . Comparison of three different MR perfusion techniques and MR spectroscopy for multiparametric assessment in distinguishing recurrent high-grade gliomas from stable disease. Acad Radiol 2013; 20: 1557–65. doi: 10.1016/j.acra.2013.09.003 [DOI] [PubMed] [Google Scholar]
- 45.Kim HS, Kim J-H, Kim S-H, Cho K-G, Kim SY. Posttreatment high-grade glioma: usefulness of peak height position with semiquantitative MR perfusion histogram analysis in an entire contrast-enhanced lesion for predicting volume fraction of recurrence. Radiology 2010; 256: 906–15. doi: 10.1148/radiol.10091461 [DOI] [PubMed] [Google Scholar]
- 46.Tsien C, Galbán CJ, Chenevert TL, Johnson TD, Hamstra DA, Sundgren PC, et al. . Parametric response map as an imaging biomarker to distinguish progression from pseudoprogression in high-grade glioma. Journal of Clinical Oncology 2010; 28: 2293–9. doi: 10.1200/JCO.2009.25.3971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boxerman JL, Ellingson BM, Jeyapalan S, Elinzano H, Harris RJ, Rogg JM, et al. . Longitudinal DSC-MRI for Distinguishing Tumor Recurrence From Pseudoprogression in Patients With a High-grade Glioma. Am J Clin Oncol 2014;. [DOI] [PubMed] [Google Scholar]
- 48.Suh CH, Kim HS, Choi YJ, Kim N, Kim SJ. Prediction of pseudoprogression in patients with glioblastomas using the initial and final area under the curves ratio derived from dynamic contrast-enhanced T1-weighted perfusion MR imaging. American Journal of Neuroradiology 2013; 34: 2278–86. doi: 10.3174/ajnr.A3634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yun TJ, Park CK, Kim TM, Lee SH, Kim JH, Sohn CH, et al. . Glioblastoma treated with concurrent radiation therapy and temozolomide chemotherapy: differentiation of true progression from pseudoprogression with quantitative dynamic contrast-enhanced MR imaging. Radiology 2015; 274: 830–40. doi: 10.1148/radiol.14132632 [DOI] [PubMed] [Google Scholar]
- 50.Shin KE, Ahn KJ, Choi HS, Jung SL, Kim BS, Jeon SS, et al. . DCE and DSC MR perfusion imaging in the differentiation of recurrent tumour from treatment-related changes in patients with glioma. Clin Radiol 2014; 69: e264–e272. doi: 10.1016/j.crad.2014.01.016 [DOI] [PubMed] [Google Scholar]
- 51.Leu K, Boxerman JL, Ellingson BM. Effects of MRI protocol parameters, preload injection dose, fractionation strategies, and leakage correction algorithms on the fidelity of dynamic-susceptibility contrast MRI estimates of relative cerebral blood volume in gliomas. AJNR Am J Neuroradiol 2017; 38: 478–84. doi: 10.3174/ajnr.A5027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Law M, Young R, Babb J, Pollack E, Johnson G. Histogram analysis versus region of interest analysis of dynamic susceptibility contrast perfusion MR imaging data in the grading of cerebral gliomas. AJNR American journal of neuroradiology 2007; 28: 761–6. [PMC free article] [PubMed] [Google Scholar]
- 53.LS H, Baxter LC, Pinnaduwage DS, Paine TL, Karis JP, Feuerstein BG, et al. . Optimized preload leakage-correction methods to improve the diagnostic accuracy of dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging in posttreatment gliomas. AJNR American journal of neuroradiology 2010; 31: 40–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kudo K, Uwano I, Hirai T, Murakami R, Nakamura H, Fujima N, et al. . Comparison of different post-processing algorithms for dynamic susceptibility contrast perfusion imaging of cerebral gliomas. Magnetic Resonance in Medical Sciences 2017; 16: 129–36. doi: 10.2463/mrms.mp.2016-0036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kalpathy-Cramer J, Gerstner ER, Emblem KE, Andronesi OC, Rosen B. Advanced magnetic resonance imaging of the physical processes in human glioblastoma. Cancer Res 2014; 74: 4622–37. doi: 10.1158/0008-5472.CAN-14-0383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ellingson BM, Malkin MG, Rand SD, Connelly JM, Quinsey C, LaViolette PS, et al. . Validation of functional diffusion maps (fDMs) as a biomarker for human glioma cellularity. Journal of Magnetic Resonance Imaging 2010; 31: 538–48. doi: 10.1002/jmri.22068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ellingson BM, Malkin MG, Rand SD, LaViolette PS, Connelly JM, Mueller WM, et al. . Volumetric analysis of functional diffusion maps is a predictive imaging biomarker for cytotoxic and anti-angiogenic treatments in malignant gliomas. J Neurooncol 2011; 102: 95–103. doi: 10.1007/s11060-010-0293-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moffat BA, Chenevert TL, Lawrence TS, Meyer CR, Johnson TD, Dong Q, et al. . Functional diffusion map: A noninvasive MRI biomarker for early stratification of clinical brain tumor response. Proceedings of the National Academy of Sciences 2005; 102: 5524–9. doi: 10.1073/pnas.0501532102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ellingson BM, Cloughesy TF, Lai A, Nghiemphu PL, Liau LM, Pope WB. Quantitative probabilistic functional diffusion mapping in newly diagnosed glioblastoma treated with radiochemotherapy. Neuro Oncol 2013; 15: 382–90. doi: 10.1093/neuonc/nos314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pope WB, Qiao XJ, Kim HJ, Lai A, Nghiemphu P, Xue X, et al. . Apparent diffusion coefficient histogram analysis stratifies progression-free and overall survival in patients with recurrent GBM treated with bevacizumab: a multi-center study. J Neurooncol 2012; 108: 491–8. doi: 10.1007/s11060-012-0847-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lutz K, Wiestler B, Graf M, Bäumer P, Floca R, Schlemmer H-P, et al. . Infiltrative patterns of glioblastoma: Identification of tumor progress using apparent diffusion coefficient histograms. Journal of Magnetic Resonance Imaging 2014; 39: 1096–103. doi: 10.1002/jmri.24258 [DOI] [PubMed] [Google Scholar]
- 62.Zeng Q, Dong F, Shi F, Ling C, Jiang B, Zhang J. Apparent diffusion coefficient maps obtained from high b value diffusion-weighted imaging in the preoperative evaluation of gliomas at 3T: comparison with standard b value diffusion-weighted imaging. Eur Radiol 2017; 27: 5309–15. doi: 10.1007/s00330-017-4910-0 [DOI] [PubMed] [Google Scholar]
- 63.Wang Q, Zhang H, Zhang J, Wu C, Zhu W, Li F, et al. . The diagnostic performance of magnetic resonance spectroscopy in differentiating high-from low-grade gliomas: A systematic review and meta-analysis. Eur Radiol 2016; 26: 2670–84. doi: 10.1007/s00330-015-4046-z [DOI] [PubMed] [Google Scholar]
- 64.van Dijken BRJ, van Laar PJ, Holtman GA, van der Hoorn A. Diagnostic accuracy of magnetic resonance imaging techniques for treatment response evaluation in patients with high-grade glioma, a systematic review and meta-analysis. Eur Radiol 2017; 27: 4129–44. doi: 10.1007/s00330-017-4789-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang W, Hu Y, Lu P, Li Y, Chen Y, Tian M, et al. . Evaluation of the diagnostic performance of magnetic resonance spectroscopy in brain tumors: a systematic review and meta-analysis. PLoS One 2014; 9: e112577. doi: 10.1371/journal.pone.0112577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Choi C, Ganji SK, DeBerardinis RJ, Hatanpaa KJ, Rakheja D, Kovacs Z, et al. . 2-hydroxyglutarate detection by magnetic resonance spectroscopy in IDH-mutated patients with gliomas. Nat Med 2012; 18: 624–9. doi: 10.1038/nm.2682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Andronesi OC, Rapalino O, Gerstner E, Chi A, Batchelor TT, Cahill DP, et al. . Detection of oncogenic IDH1 mutations using magnetic resonance spectroscopy of 2-hydroxyglutarate. J Clin Invest 2013; 123: 3659–63. doi: 10.1172/JCI67229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhou M, Zhou Y, Liao H, Rowland BC, Kong X, Arvold ND, et al. . Diagnostic accuracy of 2-hydroxyglutarate magnetic resonance spectroscopy in newly diagnosed brain mass and suspected recurrent gliomas. Neuro Oncol 2018; 20: 1262–71. doi: 10.1093/neuonc/noy022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Choi C, Raisanen JM, Ganji SK, Zhang S, McNeil SS, An Z, et al. . Prospective longitudinal analysis of 2-hydroxyglutarate magnetic resonance spectroscopy identifies broad clinical utility for the management of patients with IDH -mutant glioma. Journal of Clinical Oncology 2016; 34: 4030–9. doi: 10.1200/JCO.2016.67.1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.de la Fuente MI, Young RJ, Rubel J, Rosenblum M, Tisnado J, Briggs S, et al. . Integration of 2-hydroxyglutarate-proton magnetic resonance spectroscopy into clinical practice for disease monitoring in isocitrate dehydrogenase-mutant glioma. Neuro Oncol 2016; 18: 283–90. doi: 10.1093/neuonc/nov307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Basu S, Alavi A. Molecular Imaging (PET) of Brain Tumors. Neuroimaging Clin N Am 2009; 19: 625–46. doi: 10.1016/j.nic.2009.08.012 [DOI] [PubMed] [Google Scholar]
- 72.Colavolpe C, Chinot O, Metellus P, Mancini J, Barrie M, Bequet-Boucard C, et al. . FDG-PET predicts survival in recurrent high-grade gliomas treated with bevacizumab and irinotecan. Neuro Oncol 2012; 14: 649–57. doi: 10.1093/neuonc/nos012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Galldiks N, Langen K-J. Amino acid PET in neuro-oncology: applications in the clinic. Expert Rev Anticancer Ther 2017; 17: 395–7. doi: 10.1080/14737140.2017.1302799 [DOI] [PubMed] [Google Scholar]
- 74.Verger A, Arbizu J, Law I. Role of amino-acid PET in high-grade gliomas: limitations and perspectives. The quarterly Journal of Nuclear Medicine and Molecular Imaging 2018; 62. doi: 10.23736/S1824-4785.18.03092-3 [DOI] [PubMed] [Google Scholar]
- 75.Albert NL, Weller M, Suchorska B, Galldiks N, Soffietti R, Kim MM, et al. . Response assessment in neuro-oncology working group and european association for neuro-oncology recommendations for the clinical use of PET imaging in gliomas. Neuro Oncol 2016; 18: 1199–208. doi: 10.1093/neuonc/now058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wakabayashi T, Iuchi T, Tsuyuguchi N, Nishikawa R, Arakawa Y, Sasayama T, et al. . Diagnostic performance and safety of positron emission tomography using (18)F-fluciclovine in patients with clinically suspected high- or low-grade gliomas: A multicenter phase IIb trial. Asia Ocean J Nucl Med Biol 2017; 5: 10–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sasikumar A, Kashyap R, Joy A, Charan Patro K, Bhattacharya P, Reddy Pilaka VK, et al. . Utility of 68Ga-PSMA-11 PET/CT in Imaging of Glioma-A Pilot Study. Clin Nucl Med 2018; 43: e304–e309. doi: 10.1097/RLU.0000000000002175 [DOI] [PubMed] [Google Scholar]
- 78.Erickson BJ, Korfiatis P, Akkus Z, Kline TL. Machine learning for medical imaging. Radiographics 2017; 37: 505–15. doi: 10.1148/rg.2017160130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hu X, Wong KK, Young GS, Guo L, Wong ST. Support vector machine multiparametric MRI identification of pseudoprogression from tumor recurrence in patients with resected glioblastoma. Journal of Magnetic Resonance Imaging 2011; 33: 296–305. doi: 10.1002/jmri.22432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jang B-S, Jeon SH, Kim IH, Kim IA. Prediction of pseudoprogression versus progression using machine learning algorithm in glioblastoma. Sci Rep 2018; 8: 12516. doi: 10.1038/s41598-018-31007-2 [DOI] [PMC free article] [PubMed] [Google Scholar]