Abstract
Objective:
To evaluate high-precision external beam reirradiation (re-EBRT) for local relapse of prostate cancer (PCa) after radiotherapy.
Methods:
This retrospective study included patients with biochemical failure and evidence of isolated local recurrence of PCa after radical/salvage EBRT or brachytherapy that received salvage stereotactic body radiation therapy (SBRT, re-EBRT). Biopsy was not mandatory if all diagnostic elements were univocal (prostate specific antigen evolution, choline-positron emission tomography or magnetic resonance imaging). Salvage SBRT (re-EBRT) was delivered with image-guided radiation therapy (RapidArc®, VERO® and CyberKnife®).
Results:
Data of 64 patients were included, median age at salvage SBRT was 73.2 years, median pre-salvage SBRT prostate specific antigen was 3.89 ng ml−1 . Median total dose was 30 Gy in five fractions, biologically effective dose (BED) of 150 Gy. One acute G3 genitourinary event and one late G3 genitourinary event were observed. No G ≥ 3 bowel toxicity was registered. At the median follow-up of 26.1 months, tumor progression was observed in 41 patients (64%). 18 patients (28%) experienced local relapse. 2-year local control, biochemical and clinical relapse free survival rates were 75, 40 and 53%, respectively. With BED ≥130 Gy 1-year biochemical and clinical progression-free survival rate were 85 and 90%, respectively.
Conclusions:
Salvage SBRT (re-EBRT) for isolated local PCa recurrence is a safe, feasible and noninvasive salvage treatment. Further investigation is warranted to define the optimal patient selection, dose and volume parameters.
Advances in knowledge:
Salvage SBRT reirradiation for the locally recurrent PCa offer a satisfactory tumor control and excellent toxicity profile, if BED ≥130 Gy is administered.
INTRODUCTION
External beam radiotherapy (EBRT) is widely used as primary treatment for localized prostate cancer (PCa).1 A recent randomized study with a median 10 years follow-up demonstrated that, in localized low and intermediate risk PCa, EBRT achieves results similar to radical prostatectomy in terms of tumor control and disease specific mortality.2 In recent years, the development of modern techniques such as intensity modulated radiation therapy (IMRT), image-guided radiation therapy (IGRT) and stereotactic body irradiation (SBRT), in addition to the progressive escalation of the prescribed dose, resulted in good tumor local control and enhanced overall survival in patients affected by localized PCa.3,4 However, still approximately 22–69% of patients develop biochemical failure after radical EBRT or radical prostatectomy.5,6
Currently, the standard treatment for locally recurrent PCa is not uniquely defined7,8 and different therapeutic options are available, including systemic therapy, i.e. androgen deprivation therapy (ADT), or local salvage approaches with curative intent, such as salvage prostatectomy, re-irradiation, cryotherapy and high intensity focused ultrasound (HIFU). The majority of the patient series is small and includes heterogeneous patients and treatments without a long follow-up. No randomized trials comparing salvage local and systemic strategies are available.7,9,10 The most effective local therapy has not been established yet and remains controversial in the absence of consensus.11–13 At present, patients with biochemical failure mainly receive longlasting ADT with negative impact on the quality of life and other health aspects. In selected patients with prostate specific antigen (PSA) <10 ng ml−1 and a good life expectancy (>10 years) a local treatment with radical intent can be considered.7,12
As far as reirradiation is concerned, the vast majority of patient series were treated with brachytherapy (BRT). In the last years, along with the improvement in radiotherapy (RT) planning and delivery technology, several reports on salvage EBRT have been published14–27 (Table 1).
Table 1.
Published clinical series including patients treated with external beam reirradiation for locally recurrent prostate cancer>.>
| Author, year of publication [Ref] | N | Initial treatment modality | Median relapse time (range) | Treated volume | RT technique | Total Dose and fractionation | ADT | Median follow-up (range) | Toxicity | Local control/ pattern of failure | Overall survival |
| Kalapurakal, 200324 | 13 |
Group A: EBRT ± ADT: 8 pts Group B: ADT : 4 pts prostatectomy + orchiectomy: 1 |
Not reported | PTV: GTV + 1–2 cm | 3D-CRT + hyperthermia | Median total dose 39.6 Gy/ 22 fr (prior RT) Median total dose 66.6 (59.4–70.2) fr (no prior RT) |
Neoadjuvant: 1 pts |
Group A: 14 (4–48) months Group B: 15 (6–32) months |
Acute: Grade 4: 1 GI Late Grade 1: 3 GI, 1 GU Grade 2: 1 GI Grade 4: 2 GU |
Group A: Median duration of CR/PR: 12 (4-27) months Group B Median duration of CR/PR: 15 (2-32) months |
N.A. |
| Vavassori, 201015 |
6 | EBRT | Not reported | Whole gland | SBRT | 30 Gy/ 5 fr | Neoadjuvant: 4 pts | 11.2 (9.6–18.6) months |
Acute: Grade > 2: 0 pts Late Grade > 2: 0 pts |
Median time to clinical progression: 9.9 (9.2–10) months (1 regional, 2 distant) | 11.2 months OS: 100% |
| Jereczek-Fossa, 201219 |
34 pts/38 lesions | EBRT ± ADT: 20 pts Radical prostatectomy ±RT ± ADT: 14 pts |
66 (24–180) months | Local recurrence (15 pts) Anastomosis recurrence (4 pts) Lymph node recurrence (16 lesions) Metastasis (3 lesion) |
SBRT |
Local recurrence Median total dose 30 Gy/4.5 fr Anastomosis recurrence Median total dose 30 Gy/5 fr |
Concomitant, 5 pts Concomitant, 2 pts |
9.5 (3–28.9) months 23 (3.9–30.6) months |
Local and anastomosis recurrence Acute: Grade 1: 3 GU, 1 GI Grade 2: 2 GU Grade 3: 1 GU Late: Grade 1: 1 GU Grade 2: 1 GU, 1 GI Grade 3: 1 GU |
30 months PFS (%) (95% CI): 22.2 (0–58.2) Median PFS (%) (95% CI):: 13 (10, > 30) 30 months PFS (%) (95% CI) : 33.0 (0–68.7) Median PFS (%) (95% CI): 14 (10, > 30) |
Not reported |
| Zerini, 201514 |
32 | EBRT ± ADT: 10 pts Radical prostatectomy ±RT ± ADT : 22 pts |
99.7 (23–208.4) months | Whole gland (22 pts) Prostate bed (10 pts) |
SBRT | Median total dose 25 Gy/ median dose/fr 5 Gy (if no prior surgery); median total dose 25 Gy/ 5 fr (if prior surgery) | Concomitant, 11 pts | 21.3 (2–53) months |
Acute: Grade 1: 6 GU, 2 GI Grade 2: 2 GU, 1 GI Late: Grade 1: 6 GU Grade 2: 1 GU |
Median time to biochemical progression: 9.4 (4.9–27.8) months Median time to clinical progression: 13.2 (2–53) months (4 local, 1 regional, 7 metastatic) |
21.3 months cancer-specific OS: 93.7% |
| Fuller, 201516 | 29 | EBRT | 88 (32–200) months | Whole gland | SBRT | 34 Gy/5 fr | No | 24 (3–60) months |
Late Grade 2: 3 Grade 3: 1 Grade 4: 1 |
Actuarial 2 year BFFS: 82% 2 year DFS: 100% |
2 year OS: 100% |
| Arcangeli, 201525 | 1 | Radical prostatectomy +RT | 42 months | PTV: CTV + 5 mm | SBRT | 30 Gy/5 fr | No | 6 months |
Acute: Grade 1 GU, Late: Grade 0 |
6 months LC: 100% 6 months BFFS: 100% |
6 months: 100% |
| Lee, 201527 | 2 | EBRT | Case1: 102 months Case 2: 35 months |
Whole gland | IMRT | Case 1: 66 Gy/30 fr Case 2: 69 Gy/30 fr |
no | Case 1: not reported Case 2: 4 years |
Acute: Grade > 3: 0, Late: Grade > 3: 0 |
Not reported | Not reported |
| Zilli, 201622 | 14 | EBRT: 12 pts Primary EBRT ±BRT boost: 2 pts |
4.4 (2.3–7.4) years | Whole gland | 3D-CRT (10 pts) IMRT (4 pts) boost BRT (10 pts) boot EBRT (3 pts) |
Median normalized dose in 2 Gy fr: 85.1 (70–93.4) Gy, α/β: 1.5 Gy | 12 months (median time) ADT: 12 pts | 94 (48–172) months |
Acute: Grade 1: 4 GU, 6 GI Grade 2: 3 GU, 2 GI Late Grade 1: 3 GU, 1 GI Grade 2: 3 GU, 3 GI Grade 3: 4 GU, 4 GI Grade 4: 4 GU, 5 GI |
5 year BFFS: 35.7±12.8% 5 year LRFS: 50.0±13.4% 5 year DMFS: 85.7±9.4% |
5 year cancer-specific survival: 100% |
| Rutenberg, 201621 | 11 | Prostate BRT | 49.2 (12.9–135.5) months | Whole pelvis: 8 pts Prostate +proximal SVs: 1 Prostate only: 2 |
3D-CRT (2 pts), IMRT (9 pts); | median total dose: 70.2 (64.8–75.6) Gy | 6 months ADT: 3 pts 2 year ADT: 2 pts |
26.5 (1–53.6) months |
Acute: G2: 1 Late: Grade 2: 2 Grade 3: 2 |
Actuarial 3 year BFFS: 69% Median time to local relapse: 17.7 months (4 pts) |
Actuarial 3 year OS: 77% |
| Janoray, 201617 | 21 | Radical prostatectomy +RT: 10 pts Primary RT only: 11 pts |
111 (38–398) months | CTV: GTV + 1 or 2 mm per D’Amico risk stratification (low/intermediate vs high) | SBRT | 36.25 Gy/5 fr to the 80% isodose line (95% PTV coverage) | Concomitant (2 pts) | 11.7 (2.5–46.5) months |
Acute Grade 2: 1 |
1 year BFFS: 83.3% 1 year LR: 95.2% (1 pt, in field) |
11.7 months OS: 100% |
| Detti, 201618 | 16 | Radical prostatectomy: 8 pts Radical prostatectomy +RT: 8 pts |
10.5 (3.5–21.4) years | Prostatic bed | SBRT | 30 Gy/5 fr (if previous RT); 35 Gy/5 fr (if no previous RT) |
Adjuvant (5 pts) | 10 (2–21) months |
Acute: Grade 1: 1 Late: G2:1 |
Biochemical response: 15 pts Median time to relapse: 9.3 months (7, distant relapse) |
10 months OS: 100% |
| Leroy, 201720 | 23 | EBRT: 19 pts BRT: 4 pts |
65 (28–150) months | Whole gland: 19 Focal: 3 Hemi-prostate: 1 |
SBRT | 36 Gy to the 80% isodose line (95% PTV coverage) | Concomitant (14 pts) | 22 (6–40) months | Time of onset: not reported Grade 1: 13 Grade 2: 9 Grade 3: 3 Grade 4: 0 |
2 year DFS: 54% 20 months DFS: 60.9% (5 local, 1 nodal and 3 metastatic recurrences) |
2 year OS: 100% |
| Mbeutcha, 201726 | 28 | HDRB: 16 pts EBRT: 12 pts |
69 months (IQR: 55–85) 49 months (IQR: 37–70) |
PTV: CTV + 1 mm | BRT (10 pts), SBRT (18 pts) |
35 Gy/5 fr 35 Gy/5 fr |
Concomitant (2 pts) Concomitant (10 pts) |
22.5 months (IQR: 8–42) 14.5 months (IQR: 7–23) |
Acute: Grade 1: 2 GU, 1 GI Grade 2: 7 GU Late: Grade 1: 1 GI Grade 2: 6 GU Grade3: 1 GU Acute: Grade 1: 5 GU, 1 GI Grade 2: 2 GU, 2 GI Late: Grade1:4 GU Grade 2: 1 GU, 1 GI Grade 4: 1 GU |
BRT: Biochemical recurrence free survival: 44.4% at 19.5 months (IQR: 14–36) SBRT: Biochemical recurrence free survival: 33.3% at 7 months (IQR: 4–7) |
N.A. |
| Loi, 201823 | 50 | Post-prostatectomy RT: 22 pts Radical EBRT: 26 pts |
76 (9–205) months | Whole gland, DIL |
SBRT | 30 Gy to the 80% isodose line (95% PTV coverage) | Concomitant (11 pts) | 21.3 (6.1–49.2) |
Acute: Grade 1: 9 GU, 4 GI Grade 2: 1 GU Grade 3: 1 GU Late: Grade 1: 9 GU, 1 GI Grade 2: 3 GU, 2 GI Grade 3: 1 GU, 1 GI |
1 year BFFS: 80% 1 year DMFS: 92% |
N.A. |
| Current study | 64a | Post-prostatectomy RT: 19 pts Radical EBRT: 40 pts Radical BRT: 4 pts Radical EBRT + BRT: 1 pts |
99.7 (23–208.4) months | PPI: 4 Whole gland: 40 Whole gland +DIL: 1 Prostate bed recurrence: 19 |
IMRT (50 pts) SBRT (14pts) |
Median total dose 30 Gy/ 6 fr, Median dose/fraction 6 Gy (3–12). Median number of fractions 5 (2–10) |
Concomitant (16 pts) | 26.1 (3.1–82.4) months |
Acute: Grade 2: 3 GU, 1 GI Grade 3: 1 GU Late: Grade 2: 6 GU, 1 GI Grade 3: 1 GU |
2 year LC: 75% 2 year BFFS: 40% 2 year Clinical free survival: 53% |
2 year OS: 92%. 2 year PCSS: 95% |
3D-CRT, 3 dimensional-conformal radiation therapy; ADT, androgen deprivation therapy; BFFS, biochemical failure free survival; BRT, brachytherapy; CR/PR, complete response/partial response; CTV, clinical target volume; DFS, disease-free survival; DIL, dominant intraprostic lesion; DMFS, distant metastases free survival; EBRT, external beam radiation therapy; fr, fraction; GTV, gross tumor volume; GTVGI, gastrointestinal; GU, genitourinary; IMRT, intensity modulated radiation therapy; LC, local control; N.A, not available; OS, overall survival; PCSS, prostate cancer specific survival; PPI, partial prostate irradiation; pt, patient; RT, radiation therapy; SBRT, stereotactic radiation therapy;
27 pts from Zerini et al14 with up-dated follow-up.
Our Division, equipped with high precision RT systems, has a long experience of reirradiation.19,28–30 Our reports include numerous primary tumor sites, and the majority of studies are focused on the recurrent PCa.14,15,19,28–34 In particular, the preliminary retrospective analysis published by Zerini et al14 with a mean follow-up of 21.3 months, reported good local tumor control and low toxicity profile in a series of 32 patients treated with IG-IMRT for isolated local recurrence of PCa. The aim of the current study was to present the outcome in the larger patient series treated with salvage SBRT (re-EBRT), including updated information of some patients from Zerini's series.14
Methods and materials
Study protocol
Inclusion criteria for this retrospective study were: (1) isolated local recurrence of PCa after primary EBRT, BRT or salvage post-prostatectomy RT; (2) salvage with SBRT (re-EBRT) at the European Institute of Oncology between November 2009 and November 2016; (3) written informed consent for radiation treatment; (4) written informed consent for use of anonymized clinical and imaging data for research and education purpose; (5) minimum follow-up of 3 months; (6) no evidence of metastatic disease. No other local salvage treatment for the recurrent PCa was permitted.
This study is a part of the research notified to the Ethical Committee of the European Institute of Oncology (notification Nr 79: clinical and dosimetric aspects of IGRT for PCa). Data of some patients were shared with the French Genito-Urinary Group (GETUG) and a separate analysis will be performed. The GETUG analysis will regard exclusively the cases with biopsy-proven intraprostatic recurrence” (unpublished data).
Biochemical failure
Biochemical failure after the primary therapy was defined as two consecutive risings in PSA level >0.2 ng ml−1 post-radical prostatectomy and PSA nadir +2 ng ml−1 above the nadir after primary RT, according to the Radiation Therapy Oncology Group (RTOG) and American Society for Radiation Oncology (ASTRO) Phoenix Consensus.35
The diagnosis of local recurrence was based on the biochemical failure confirmed by imaging studies, i.e. [11C]-choline positron emission tomography with co-registered CT (PET/CT), whole body MRI (WB-MRI) including multiparametric MRI (mpMRI) of the prostate, whole body CT scan. Choline-PET/CT and MRI were used after the diagnosis of biochemical recurrence to confirm local recurrence and choline PET/CT was also used to exclude nodal and bone metastasis.36–40 Biopsy was not mandatory41 if all diagnostic elements were univocal (PSA evolution stating for biochemical recurrence, [11C]-choline-PET/CT or MRI findings). All cases were discussed in the multidisciplinary uro-oncology tumor board.
Treatment procedures
Salvage SBRT (re-EBRT), was delivered with IGRT, employing CyberKnife® (Accuray, Inc., Sunnyvale, CA), Vero® (Mitsubishi Heavy Industries, Ltd., Japan and BrainLab AG, Feldkirchen, Germany) or RapidArc® (Varian Medical Systems, Palo Alto, CA) systems whose technical characteristics have been published elsewhere.14 Patients treated with CyberKnife had fiducial markers implanted into the target. All patients were asked to empty the bowel (oral and written instructions for diet and enema were given) and to have full urinary bladder for simulation CT and all treatment fractions. Gross tumor volume (GTV) contouring was based on the mpMRI and PET/CT co-registration. Clinical target volume (CTV) included the whole prostate or intraprostatic lesion or prostate bed recurrence with margins. The CTV to planning target volume (PTV) margins were 5 mm in all directions except for the posterior margin where 3 mm margin was added. For CyberKnife treatments and in case of short interval between two RT courses, comorbidity or unfavorable anatomy or dose volume histograms (DVH) data, margins were reduced of 1 mm. This decision was supported by the steep dose gradient that could be achieved with CyberKnife and intra fraction organ motion control performed in all CyberKnife treatments. The organs at risk (OARs) included rectum (and its posterior part), urinary bladder, penile bulb, penis, testis, femoral heads, peritoneal cavity and cauda equina. Dose–volume constraints were: Dose given to 30% of rectal volume <13.5 Gy; Dose given to 60% of rectal volume <6.7 Gy; Dose given to 30% of urinary bladder volume <10.6 Gy (based on our previous series data reported by Jereczek-Fossa et al).19 Extreme hypofractionated schedules were employed and the fractions were delivered on alternating days. Before every fraction image guided procedures were performed (with cone beam CT in Vero and Rapidarc patients) or intrafraction control was used (CyberKnife). Additionally, automated infrared marker-based patient-positioning device integrated into the Vero system was employed (ExacTrac, BrainLab AG Feldkirchen, Germany).The patients received premedication with dexamethasone and α-blockers.
Outcome assessment
Acute and chronic toxicity was registered by a radiation oncologist according to the RTOG/European Organization for Research and Treatment of Cancer Guideline (RTOG/EORTC) during salvage SBRT (re-EBRT), and subsequently every 6–12 months after the end of salvage SBRT (re-EBRT) . Gastrointestinal (GI) and genitourinary (GU) events were registered whereas sexual dysfunction was not analysed here due to lack of baseline evaluation. Serum PSA level was tested every 3 months until any biochemical or clinical progression. In patients with a reduction or stabilization of PSA levels at follow-up, non-additional radiological or nuclear medicine evaluation was requested.
Likewise, the primary treatment, biochemical failure after reirradiation was defined as PSA nadir +2 ng ml−1 (Phoenix consensus).35 In post-prostatectomy patients, biochemical progression was defined as a continuous increase in PSA over the pre-re-EBRT value confirmed by at least two tests.14 In case of local relapse, the data were censored for toxicity in order to avoid the misinterpretation of local symptoms of relapse as GI and GU events. Biochemical progression-free survival was measured as the time from the beginning of salvage SBRT (re-EBRT) to the PSA increase after salvage SBRT (re-EBRT). Clinical progression-free survival was measured as the time from the beginning of re-EBRT to the radiological detection of local progression or distant disease. LC was measured from the beginning of salvage SBRT (re-EBRT) and the radiological diagnosis of in-field relapse.
In patients treated by ADT and salvage SBRT (re-EBRT), the PSA value before the start of ADT was considered as pre-salvage SBRT (re-EBRT) PSA level. The prescribed dose of reirradiation was converted to biologically effective dose (BED) calculated using an α/β ratio 1.5 Gy.
Statistical analysis
Patient and tumor characteristics were represented as frequencies and percentages when classified with categorical variables and with median values and range for continuous variables.42 The correlation between treatment doses and clinical outcome were investigated with Cox proportional-hazards regression. Survival analysis was performed with Kaplan–Meier approach, and differences between groups were evaluated with log-rank test.43 A p-value < 0.05 was considered significant.
Results
Patient data
Between November 2009 and November 2016, 73 patients with biochemical failure and evidence of isolated local relapse of PCa after radical/salvage EBRT or BRT were treated with salvage SBRT (re-EBRT) in our department. For this retrospective analysis, 64 patients were eligible according the inclusion criteria. Nine patients were excluded due to metastatic disease at the time of reirradiation and one patient was excluded for the reirradiation technique (three-dimensional conformal radiotherapy, 3D-CRT). 27 out of 32 patients included in Zerini's series14 fulfilled the criteria of the current study and their updated follow-up data have been included here. The median follow-up for the whole series was 26.1 months (range 3.1–82.4 months).
Patient and tumor characteristics
Patient and tumor characteristics are listed in Table 2. Local relapse was documented by [11C]-choline PET/CT, pelvic MRI, and total body CT scan in 53 (83%), 40 (63%) and 4 (6%) patients, respectively. Biopsy of the radiologically documented recurrent lesion was performed in 28 patients (44%). For the remaining 41 patients (non-biopsied or non-positive at biopsies), diagnosis of isolated local recurrence of prostate cancer was based on PSA levels (stating for biochemical recurrence) and radiological confirmation with PET, CT scan and/or MRI. All cases were discussed with the multidisciplinary uro-oncology tumor board and the clinical decision was taken jointly.
Table 2. .
Patient characteristics (N = 64 patients)
| Characteristics | All patients, n = 64 | Prostate, n = 45 | Prostate bed, n = 19 |
| PRIOR RT | |||
| Initial PSA [ng ml–1], median (range) | 11.4 (0.5–228.5) | 11.69 (3.4–228.5) | 16.7 (0.5–110) |
| Initial Gleason Score, median (range) | 7 (2–9) | 6 (4–8) | 7 (2–9) |
| Prior RT modality | |||
| 3D | 55 | 38 | 17 |
| 3D + BRT | 1 | 1 | |
| BRT | 4 | 4 | |
| IMRT | 4 | 2 | 2 |
| Dose (Gy), median (range) | 70.2 (45–145) | 75 (50–145) | 70 (45–77.4) |
| Interval between first RT and re–EBRT [months], median (range) | 99.7 (23–208.4) | 102.6 (23–208.4) | 93.9 (27.9–183.3) |
| Re–EBRT | |||
| Age at re–EBRT [years], median (range) | 73.2 (52.6–81.7) | 65.2 (47–81.7) | 59.4 (48.8–70.5) |
| Pre re–EBRT PSA [ng/ml], median (range) | 3.89 (0.17–51.8) | 4.29 (0.24–21) | 3 (0.17–51.8) |
| Androgen deprivation | |||
| Yes (%) | 16 (25%) | 10 | 6 |
| No (%) | 48 (75%) | 35 | 13 |
| Duration [months], median (range) | 17.8 (3.0–38.1) | 14.7 (7.6–37.9) | 20.6 (3.0–38.1) |
| Biopsy of the target lesion | |||
| Yes (%) | 28 (44%) | 21 | 7 |
| Positive | 23 | 18 | 5 |
| Gleason scorea (range) | 7 (6–9) | 7 (6–9) | |
| No (%) | 36 (56%) | 24 | 12 |
| Histological +/–Radiological diagnosis | |||
| Biopsy + PET + MRI | 11 | ||
| Biopsy + MRI | 6 | ||
| Biopsy + PET | 11 | ||
| MRI + PET | 15 | ||
| PET only | 12 | ||
| PET+CT+MRI | 3 | ||
| PET+CT | 1 | ||
| MRI only | 5 | ||
| Target lesion | |||
| Prostate | 45 (70%) | 45 (70%) | |
| PPI | 4 40 | 4 40 | |
| Whole gland | 1 | 1 | |
| Whole gland+ Intraprostatic relapse | 19 (30%) | 19 (30%) | |
| Prostate bed recurrence | |||
| Total dose [Gy], median (range) | 30 (20–30) | 30 (20–30) | 25 (25–30) |
| Dose/fraction [Gy], median (range) | 6 (3–12) | 6 (3–12) | 5 (5–6) |
| Number of fractions, median (range) | 5 (2–10) | 5 (2–10) | 5 |
3D, three-dimensional conformal RT; BRT, brachytherapy; CT, whole body computer tomography; PPI, partial prostate irradiation; PET, [11C]-choline positron emission tomography with co-registered computed tomography; PPI, partial prostateirradiation; PSA, prostate specificantigen; re-EBRT, external beam re-irradiation; RT, radiotherapy; IMRT, intensity modulated radiation therapy;
available in 10 patients
Previous EBRT included 3D-CRT in 55 patients (median dose: 70.2 Gy), IMRT in 4 patients (median dose: 66.1 Gy) and 4 patients received low dose rate interstitial BRT (median dose: 145 Gy). At the first EBRT, the most of the patients were treated with a conventional fractionation, and 9 patients received moderate hypofractionation.
Treatment
Salvage SBRT (re-EBRT) was performed for intraprostatic recurrence and for post-prostatectomy bed recurrence in 45 (70%) and 19 (30%) cases, respectively. CTV of reirradiation included the whole prostate in 40 patients (63%), mpMRI-identified intraprostatic relapse (partial prostate reirradiation) in 4 patients (6%), whole prostate and simultaneous boost to intraprostatic relapse in 1 patient (1%) and prostatic surgical bed nodule in 19 patients (30%). The schedules used for salvage SBRT (re-EBRT)are presented in the Table 3.
Table 3. .
Treatment schedules
|
Total dose [Gy] (Dose/fraction [Gy] × num. fractions) |
30 (3 × 10) | 25 (5 × 5) |
30 (5 × 6) | 30 (6 × 5) | 20 (10 × 2) | 24 (12 × 2) | |
| BED [Gy] (α/β = 1.5 Gy) | 90 | 108.3 | 130 | 150 | 153.3 | 216 | |
| LINAC | Total (%) | Number of patients | |||||
| CyberKnife® | 3 (5%) | – | 1 | – | – | 1 | 1 |
| VERO® | 54 (84%) | 3 | 18 | 1 | 28 | – | – |
| Trilogy® (RAPIDARC) | 7 (11%) | – | 8 | – | 3 | – | – |
BED: biologically effective doseIMRT, intensity modulated radiation therapy;
Extreme hypofractionation was employed in the majority of patients. Median dose was 30 Gy (range: 20–30 Gy) given in five fractions (range: 2–10). The choice of the schedule was based on the clinical situation (age, comorbidity, time interval between two RT courses ìetc.) and was at the physician discretion. In three patients, hypofractionated SBRT was employed due to important comorbidity
Patients treated with a total salvage SBRT (re-EBRT) BED <130 Gy had major comorbidities (i.e. ischemic cardiopathy, previous percutaneous transluminal coronary angioplasty), previous abdominal surgery and antiplatelet/anticoagulant therapy.
Concomitant ADT included luteinizing hormone-releasing hormone agonist (LHRHa), antiandrogens and combined androgen blockade (CAB) in 8, 4 and 4 patients, respectively.
Tumor outcome
At the median follow-up of 26.1 months from salvage SBRT (re-EBRT) (range: 3.1–82.4 months), progressive disease was observed in 41 patients (64%) (Table 4). In all cases, clinical progression followed biochemical progression. 18 patients (28%) experienced clinically/radiologically evident local relapse. Median time to progression was 14 months (range: 3.1–65.9 months), which was similar in both groups, namely 13.8 months (range 3.4–51.8) for prostate-bed subgroup and 14 months (range 3.1–65.3) for prostate subgroup. Patients irradiated at prostate-bed appear to have higher proportion of clinical relapse, whether patients treated on prostate presented a higher proportion of biochemical recurrence only. However, differences between groups were not statistically significant at χ2 test (we added these results in Table 4).
Table 4. .
Patterns of failure evaluated on 41 patients
| Outcome | All patients (%) | Prostate | Prostate bed | p-value |
| Biochemical relapse only | 11 (27%) | 9 (33%) | 2 (14%) | 0.19 |
| Whole gland | 8 | |||
| PPI | 1 | |||
| Concomitant ADT | 3 | 2 | 1 | |
| Clinical recurrence IN-FIELDa | 13 (32%) | 8 (30%) | 5 (36%) | 0.69 |
| Concomitant ADT | 5 | 3 | 2 | |
| Clinical recurrence IN-FIELD and OUT-FIELDb | 5 (12%) | 2 (7%) | 3 (21%) | 0.19 |
| Concomitant ADT | 2 | 0 | 2 | |
| Clinical recurrence OUT-FIELDa | 12 (29%) | 8 (29%) | 4 (29%) | 0.94 |
| Locoregional relapse with metastatic relapse | 2 (5%) | 1 (4%) | 1 (7%) | .62 |
| Concomitant ADT | 0 | 0 | 0 | |
| Locoregional relapse | 5 (12%) | 5 (18%) | 0 | – |
| Concomitant ADT | 1 | 1 | 0 | |
| Metastatic relapse | 5 (12%) | 2 (7%) | 3 (21%) | 0.19 |
| Concomitant ADT | 1 | 1 | 0 | |
| TOTAL | 41 | 27 | 14 |
ADT: Androgen deprivation therapy PPI: partial prostate irradiation
Medianfollow-up was 26.1 months. Statisticalanalysis is performed with χ2 test.
And biochemical relapse
With biochemical relapse and metastatic relapse out-field
The 2 year actuarial biochemical progression-free survival and clinical progression free survival rates were 40 and 53%, respectively. Local control at 2 years was 75%. Overall survival and PCa specific survival rates at 2 years were 92 and 95%, respectively. Five patients with a second clinical and biochemical relapse underwent a new re-EBRT.44 At the last follow-up, 59 patients were alive. 23 (36%) patients showed no evidence of disease, 35 patients (44%) were alive with biochemical or clinical disease and 1 was lost to follow-up. Five patients (8%) died: three for disease progression, one for another type of tumor and one of unknown cause.
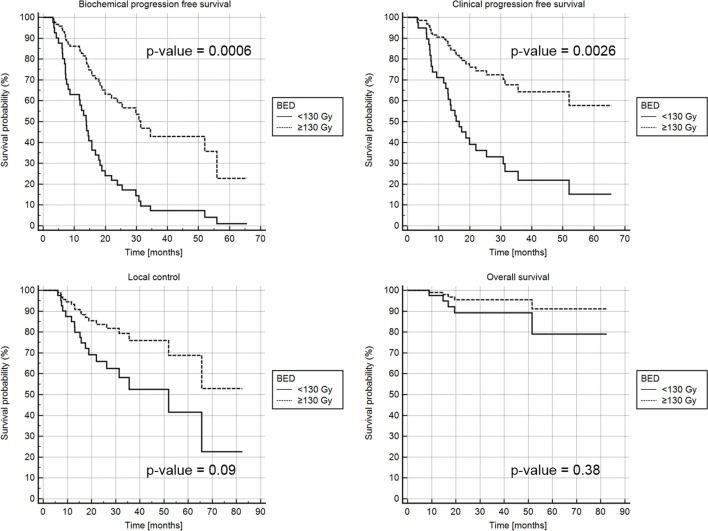
Considering salvage SBRT (re-EBRT)BED (≥130 Gy vs <130), statistically significant differences were found for the 1-year biochemical progression-free survival rate (85 vs 60%, p-value = 0.0006) and 1-year clinical progression-free survival rate (90 vs 73%, p-value = 0.0026), as shown in the Kaplan–Meier curves (Figure 1). No statistically significant differences between patients treated with a total BED <130 Gy or ≥130 Gy were found for local control at 2 years (85% vs 65%, p-value = 0.09) and overall survival at 2 years (95% vs 90%, p-value = 0.38).
Figure 1. .
(a) b-PFS rate, (b) c-PFS rate, (c)LC, (d) OS by BED <130 Gy (solid line) and BED ≥130 Gy (dashed line). BED,biologically effective dose; b-PFS, Biochemical progression free survival rate; c-PFS, Clinical progression free survival rate; LC, local control; OS, Overall survival.
For patients treated with addition of ADT and without ADT, biochemical progression was observed in 12/16 patients (75%) and in 25/48 (52%) patients, respectively.
Considering salvage SBRT (re-EBRT) setting (prostate in place and post-prostatectomy tumor bed), tumor progression was observed in 27/45 patients (60%) and 14/19 (74%) patients, respectively.
Toxicity
Acute toxicity was assessed in 64 patients (Table 5). Considering the maximum grade of toxicity observed at the end or during the first 6 months after salvage SBRT (re-EBRT), 46 patients (72%) had no acute toxicity. One patient experienced acute Grade 3 GU event represented by transitory macroscopic hematuria.
Table 5. .
Acute and late toxicity. Genito-urinary and gastro-intestinal toxicity is presented for all patients and for prostate and prostate-bed subgroups. Statistical analysis is performed with Chi-square test
| Acute toxicity (available in 64 patients) | ||||||||
| All patients | GU | GI | ||||||
| Grade | GU | GI | Prostate | Prostate bed | p-value | Prostate | Prostate bed | p-value |
| G0 | 46 (72%) | 58 (90%) | 31 (69%) | 15 (79%) | 0.41 | 42 (93.5%) | 16 (84%) | 0.25 |
| G1 | 13 (20%) | 5 (8%) | 11 (24.5%) | 2 (11%) | 0.21 | 2 (4.5%) | 3 (16%) | 0.12 |
| G2 | 3 (5%) | 1 (2%) | 2 (4.5%) | 1 (5%) | 0.88 | 1 (2%) | 0 | – |
| G3 | 1 (1.5%) | 0 | 1 (2%) | 0 | – | 0 | 0 | – |
| NE | 1a (1.5%) | 0 | 0 | 1 (5%) | – | 0 | 0 | – |
| Total patients | 64 | 64 | 45 | 19 | 45 | 19 | ||
| Late toxicity (available in 62 patients) | ||||||||
| Grade | All patients | GU | GI | |||||
| GU | Prostate | Prostate | Prostate bed | p-value | Prostate | Prostate bed | p-value | |
| G0 | 36 (57%) | 57 (89.5%) | 24 (54%) | 12 (63%) | 0.47 | 41 (91%) | 16 (84.1%) | 0.42 |
| G1 | 18 (28%) | 4 (6%) | 18 (40%) | 0 | – | 3 (7%) | 1 (5.3%) | 0.73 |
| G2 | 6 (9%) | 1 (1.5%) | 1 (2%) | 5 (26.4%) | 0.002 | 0 | 1 (5.3%) | – |
| G3 | 1 (1.5%) | 0 | 1 (2%) | 0 | – | 0. | 0 | – |
| NE | 1a (1.5%) | 0 | 0 | 1 (5.3%) | – | 0 | 0 | – |
| Missing data | 2 (3%) | 2 (3%) | 1 (2%) | 1 (5.3%) | 0.41 | 1 (2%) | 1 (5.3%) | |
| Total patients | 64 | 64 | 45 | 19 | 45 | 19 | ||
GI, gastrointestinal; GU, genitourinary; NE, Not evaluable;
Genitourinary and gastrointestinal toxicity is presented for all patients and for prostate and prostate-bed subgroups. Statistical analysis is performed with χ2 test.
toxicity not evaluable in one patient with urinary catheter positioned before reirradiation.
Late toxicity was evaluated in 62 patients (Table 5). One patient experienced late Grade 3 GU event represented by permanent reduction in urinary bladder capacity. Late toxicity was missing for two patients (follow-up <6 months). No patient developed Grade 4 or 5 toxicity.
To evaluate long-term toxicity in our series, we reviewed the data of the patients with follow-up longer than 36 months. In 20 patients monitored for more than 36 months after salvage SBRT (re-EBRT (31% of our series), only 1 Grade 3 GU event was registered (reduction in urinary bladder capacity).
No significant difference was observed between the groups with regard to acute toxicity. As concerning late toxicity, Grade 2 genitourinary events appear to be more frequent in patients who received reirradiation to prostate bed (5 events out of 19 patients in prostate-bed subgroup against 1 event out of 45 patients in prostate subgroup, p-value = 0.002, χ2 test).
Discussion
To the best of our knowledge, this is the largest series on the salvage SBRT (re-EBRT) of isolated local PCa recurrence. Our study including 64 patients showed that local salvage SBRT (re-EBRT) is safe and offers promising tumor control. Indeed, only one patient experienced late Grade 3 toxicity and almost half of the patients were free of progression and in consequence free of new therapies at 2 years after salvage SBRT (re-EBRT).
Our excellent rates of GU and GI side effects confirm the good profile of toxicity reported also by other investigators in smaller series.17,18,20,21,23,25 For example, Leroy et al20 in a series of 23 patients treated with SBRT (36 Gy in 6 fractions) did not observe Grade 4 or 5 toxicity. Importantly, in our series no patient experienced urinary incontinence, a typical complication of other salvage local therapies. Urinary incontinence has been observed in a range of 35–65% for patients treated with salvage prostatectomy, 10–40% with HIFU, 10.4% with BRT and 3–19% with cryotherapy.7,9,45,46 Salvage SBRT (re-EBRT), might be considered a valid tool in the armamentarium of local salvage approaches. Its noninvasive character represents a particular benefit in patients with contraindications to more invasive therapies, like surgery, cryoablation, HIFU or even BRT (advanced age, medical conditions including coagulopathy, severe obesity, anesthesia contraindications etc.).two-dimensional
The efficacy of salvage reirradiation for locally recurrent PCa has been evaluated by several investigations.14–19,22–26,28–34 The first report on re-EBRT by Vavassori et al15 including six cases treated by CyberKnife, showed the feasibility of reirradiation with an acceptable rate of acute and early chronic toxicity (median follow-up was 11.3 months). These preliminary findings were then confirmed by the successive series.14,16–19,28–34 All but one report concluded that salvage re-EBRT is safe.14–21,23–27 Zilli et al22 published the data of 14 patients treated with whole prostate reirradiation, with the median follow-up of 94 months, showing low acute but very high late GU and GI toxicity rates (29% of patients experienced combined Grade 4 GU/GI injury). Contrarily to the Zilli’s series, we observed only 1 Grade 3 GU event among 20 males with follow-up >36 months. This huge difference between observed toxicity profiles most probably results from differences in re-EBRT techniques. All our patients received salvage SBRT whereas in the report of Zilli et al. 71% of patients were retreated with 3D-CRT BRT boost. Moreover, the first RT course in the Zilli’s series included two-dimensional irradiation in 30% of patients, probably with important exposure of rectum and urinary bladder to high doses. These findings underline the absolute necessity to use the best available techniques and extremely careful planning when reirradiation is considered. In our practice, we employ the dosimetric constraints based on the OAR doses in the first patients treated with CyberKnife re-EBRT for isolated local recurrence. 19
The satisfactory toxicity profile in our series opens the question of dose escalation. Indeed, we observed a statistically significant difference for the biochemical progression free survival and clinical progression-free survival, as shown in the Kaplan–Meier curves. Since toxicity our series was very low despite the inclusion of numerous patients with comorbidities, we do believe that BED >130 Gy should be considered in all patients undergoing salvage SBRT (re-EBRT). Higher doses were employed by some investigators15,17,20,21,23,24,26 still maintaining acceptable toxicity level and somehow higher tumor control when compared to our findings. We do believe that these somehow suboptimal tumor control might be explained both by inclusion of patients with aggressive disease (high PSA and Gleason score at the first diagnosis and at the diagnosis of recurrent cancer) and relatively low doses prescribed in our series. For example, in the series presented by Loi et al, 1-year biochemical relapse-free survival was 80%, comparable with 85% 1-year biochemical progression free survival rate in our series when BED ≥130 Gy was administered. Nonetheless, we have to considered that most our patients received a relatively low dose at the first course of RT (median dose of 70.2 Gy), while for patients recently treated in the dose escalation era with higher BED at first treatment, the safe dose of re-EBRT still have to be defined, especially for the risk of late rectal toxicity.47
The benefit of combined use of ADT in salvage local RT is not clear. Two recent randomized trials showed a progression-free survival and/or overall survival improvement when LHRHa or bicalutamide was added to salvage post-prostatectomy RT (the benefit was greater in case of more aggressive tumors and higher pre-salvage RT PSA).48,49 However, no data are available for combined ADT and RT in the re-EBRT setting.10,45,50 In our series, concomitant ADT was prescribed in 16 (25%) patients, this percentage being lower than in other series.20,46 Whenever possible, exclusive local therapy was proposed, reserving ADT for future tumor progression. Ideally, local therapy should minimize the burden of systemic therapies and their side effects. Interestingly, lower tumor control was observed in patients treated with concomitant ADT, and this can be at least partially explained by the selection of patients in whom ADT was added to re-EBRT (i.e. high initial PSA, initial castration resistance etc.). Several questions, including addition of ADT and treated volume, must still be answered in local salvage therapy of PCa. A consensus on the salvage BRT has been recently published in order to help clinicians managing intraprostatic recurrence.13 Consensus for salvage EBRT still needs to be undertaken.
Conclusion
Salvage SBRT (Re-EBRT), is a safe, feasible and noninvasive salvage treatment for the locally recurrent PCa, offering a satisfactory tumor control and excellent toxicity profile, if BED ≥130 Gy is administered. Further prospective studies are warranted to define the optimal patient selection and establish the optimal dose and volume parameters for this particular clinical scenario.
Footnotes
Acknowledgment: This study was partially supported by Associazione Italiana per la Ricerca sul Cancro (AIRC), projects IG-14300 and IG-13218, by a research grant from Accuray Inc. entitled “Data collection and analysis of Tomotherapy and CyberKnife breast clinical studies, breast physics studies and prostate study” and by a research grant from the Fondazione IEO-CCM. We thank F. Bazzani MD, S. Ronchi MD, A. Maucieri MD, F. Golino BSc and S. Mazza BSc for their help in data collection.
Conflict of interest: The authors whose names are listed above certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants;participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
The authors Barbara Alicja Jereczek-Fossa and Damaris Patricia Rojas contributed equally to the work.
Giulia Marvaso and Delia Ciardo have contributed equally to this study and should be considered as co-last authors.
Contributor Information
Barbara Alicja Jereczek-Fossa, Email: barbara.jereczek@ieo.it.
Damaris Patricia Rojas, Email: damarojas@gmail.com.
Dario Zerini, Email: dario.zerini@ieo.it.
Cristiana Fodor, Email: cristiana.fodor@ieo.it.
Anna Viola, Email: anna.viola1985@gmail.com.
Giuseppe Fanetti, Email: giuseppe.fanetti@unimi.it.
Stefania Volpe, Email: stefania.volpe@ieo.it.
Rosa Luraschi, Email: rosa.luraschi@ieo.it.
Alessia Bazani, Email: alessia.bazani@ieo.it.
Elena Rondi, Email: elena.rondi@ieo.it.
Federica Cattani, Email: federica.cattani@ieo.it.
Andrea Vavassori, Email: andrea.vavassori@ieo.it.
Cristina Garibaldi, Email: cristina.garibaldi@ieo.it.
Sarah Alessi, Email: sarah.alessi@ieo.it.
Paola Pricolo, Email: paola.pricolo@ieo.it.
Giuseppe Petralia, Email: giuseppe.petralia@ieo.it.
Gabriele Cozzi, Email: gabriele.cozzi@ieo.it.
Ottavio De Cobelli, Email: ottavio.decobelli@ieo.it.
Gennaro Musi, Email: gennaro.musi@ieo.it.
Roberto Orecchia, Email: roberto.orecchia@ieo.it.
Giulia Marvaso, Email: giulia.marvaso@ieo.it.
Delia Ciardo, Email: delia.ciardo@ieo.it.
REFERENCES
- 1.Grimm P, Billiet I, Bostwick D, Dicker AP, Frank S, Immerzeel J, et al. . Comparative analysis of prostate-specific antigen free survival outcomes for patients with low, intermediate and high risk prostate cancer treatment by radical therapy. Results from the prostate cancer results study group. BJU Int 2012; 109(Suppl 1): 22–9. doi: 10.1111/j.1464-410X.2011.10827.x [DOI] [PubMed] [Google Scholar]
- 2.Hamdy FC, Donovan JL, Lane JA, Mason M, Metcalfe C, Holding P, et al. . 10-Year Outcomes after monitoring, Surgery, or radiotherapy for localized prostate cancer. N Engl J Med 2016; 375: 1415–24. doi: 10.1056/NEJMoa1606220 [DOI] [PubMed] [Google Scholar]
- 3.Kalbasi A, Li J, Berman A, Swisher-McClure S, Smaldone M, Uzzo RG, et al. . Dose-Escalated Irradiation and Overall Survival in Men With Nonmetastatic Prostate Cancer. JAMA Oncol 2015; 1: 897–906. doi: 10.1001/jamaoncol.2015.2316 [DOI] [PubMed] [Google Scholar]
- 4.Meier R. Dose-Escalated Robotic SBRT for Stage I-II Prostate Cancer. Front Oncol 2015; 5: 48. doi: 10.3389/fonc.2015.00048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuban DA, Thames HD, Levy LB, Horwitz EM, Kupelian PA, Martinez AA, et al. . Long-term multi-institutional analysis of stage T1-T2 prostate cancer treated with radiotherapy in the PSA era. Int J Radiat Oncol Biol Phys 2003; 57: 915–28org/S0360301603006321 3. doi: 10.1016/S0360-3016(03)00632-1 [DOI] [PubMed] [Google Scholar]
- 6.Macdonald OK, Schild SE, Vora SA, Andrews PE, Ferrigni RG, Novicki DE, et al. . Salvage radiotherapy for palpable, locally recurrent prostate cancer after radical prostatectomy. Int J Radiat Oncol Biol Phys 2004; 58: 1530–5. doi: 10.1016/j.ijrobp.2003.09.082 [DOI] [PubMed] [Google Scholar]
- 7.Tetreault-Laflamme A, Crook J. Options for salvage of radiation failures for prostate cancer. Semin Radiat Oncol 2017; 27: 67–78. doi: 10.1016/j.semradonc.2016.08.007 [DOI] [PubMed] [Google Scholar]
- 8.Jereczek-Fossa BA. Re: Stereotactic Body Re-irradiation Therapy for Locally Recurrent Prostate Cancer After External-beam Radiation Therapy: Initial Report. Eur Urol 2017; 71: 144. doi: 10.1016/j.eururo.2016.08.059 [DOI] [PubMed] [Google Scholar]
- 9.Arcangeli S, Agolli L, Donato V. Retreatment for prostate cancer with stereotactic body radiation therapy (SBRT): Feasible or foolhardy? Rep Pract Oncol Radiother 2015;-; 20: 425–9. doi: 10.1016/j.rpor.2014.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alongi F, De Bari B, Campostrini F, Arcangeli S, Matei DV, Lopci E, et al. . Salvage therapy of intraprostatic failure after radical external-beam radiotherapy for prostate cancer: a review. Crit Rev Oncol Hematol 2013; 88: 550–63. doi: 10.1016/j.critrevonc.2013.07.009 [DOI] [PubMed] [Google Scholar]
- 11.Cornford P, Bellmunt J, Bolla M, Briers E, De Santis M, Gross T, et al. . EAU-ESTRO-SIOG Guidelines on prostate cancer. Part II: Treatment of relapsing, metastatic, and castration-resistant prostate cancer. Eur Urol 2017; 71: 630–42. doi: 10.1016/j.eururo.2016.08.002 [DOI] [PubMed] [Google Scholar]
- 12. National Comprehensive Cancer Network Prostate cancer. Version 2. NCCN clinical practice guidelines in oncology; 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaljouw E, Pieters BR, Kovács G, Hoskin PJ. A Delphi consensus study on salvage brachytherapy for prostate cancer relapse after radiotherapy, a Uro-GEC study. Radiother Oncol 2016; 118: 122–30. doi: 10.1016/j.radonc.2015.10.021 [DOI] [PubMed] [Google Scholar]
- 14.Zerini D, Jereczek-Fossa BA, Fodor C, Bazzani F, Maucieri A, Ronchi S, et al. . Salvage image-guided intensity modulated or stereotactic body reirradiation of local recurrence of prostate cancer. Br J Radiol 2015; 88: 20150197. doi: 10.1259/bjr.20150197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vavassori A, Jereczek-Fossa BA, Beltramo G, De Cicco L, Fariselli L, Bianchi LC, et al. . Image-guided robotic radiosurgery as salvage therapy for locally recurrent prostate cancer after external beam irradiation: retrospective feasibility study on six cases. Tumori 2010; 96: 71–5. doi: 10.1177/030089161009600112 [DOI] [PubMed] [Google Scholar]
- 16.Fuller DB, Wurzer J, Shirazi R, Bridge SS, Law J, Mardirossian G, et al. . High-dose-rate stereotactic body radiation therapy for postradiation therapy locally recurrent prostatic carcinoma: Preliminary prostate-specific antigen response, disease-free survival, and toxicity assessment. Pract Radiat Oncol 2015; 5: e615–e623. Nov-Dec. doi: 10.1016/j.prro.2015.04.009 [DOI] [PubMed] [Google Scholar]
- 17.Janoray G, Reynaud-Bougnoux A, Ruffier-Loubière A, Bernadou G, Pointreau Y, Calais G, et al. . Stereotactic body re-irradiation therapy for locally recurrent prostate cancer after external-beam radiation therapy: Initial report. Cancer Radiother 2016; 20: 275–81. doi: 10.1016/j.canrad.2016.03.005 [DOI] [PubMed] [Google Scholar]
- 18.Detti B, Bonomo P, Masi L, Doro R, Cipressi S, Iermano C, et al. . CyberKnife stereotactic radiotherapy for isolated recurrence in the prostatic bed. World J Urol 2016; 34: 311–7. doi: 10.1007/s00345-015-1613-5 [DOI] [PubMed] [Google Scholar]
- 19.Jereczek-Fossa BA, Beltramo G, Fariselli L, Fodor C, Santoro L, Vavassori A, et al. . Robotic image-guided stereotactic radiotherapy, for isolated recurrent primary, lymph node or metastatic prostate cancer. Int J Radiat Oncol Biol Phys 2012; 82: 889–97. doi: 10.1016/j.ijrobp.2010.11.031 [DOI] [PubMed] [Google Scholar]
- 20.Leroy T, Lacornerie T, Bogart E, Nickers P, Lartigau E, Pasquier D, et al. . Salvage robotic SBRT for local prostate cancer recurrence after radiotherapy: Preliminary results of the Oscar Lambret Center. Radiat Oncol 2017; 12: 95. doi: 10.1186/s13014-017-0833-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rutenberg MS, Meister M, Amin PP, Hussain A, Naslund MJ, Kwok Y, et al. . Salvage external beam radiotherapy for locally recurrent prostate cancer after definitive brachytherapy. Brachytherapy 2016; 15: 722–9. Nov - Dec. doi: 10.1016/j.brachy.2016.08.004 [DOI] [PubMed] [Google Scholar]
- 22.Zilli T, Benz E, Dipasquale G, Rouzaud M, Miralbell R. Reirradiation of prostate cancer local failures after previous curative radiation therapy: long-term outcome and tolerance. Int J Radiat Oncol Biol Phys 2016; 96: 318–22. doi: 10.1016/j.ijrobp.2016.05.024 [DOI] [PubMed] [Google Scholar]
- 23.Loi M, Di Cataldo V, Simontacchi G, Detti B, Bonomo P, Masi L, et al. . Robotic stereotactic retreatment for biochemical control in previously irradiated patients affected by recurrent prostate cancer. Clin Oncol 2018; 30: 93–100. doi: 10.1016/j.clon.2017.11.007 [DOI] [PubMed] [Google Scholar]
- 24.Kalapurakal JA, Pierce M, Chen A, Sathiaseelan V. Efficacy of irradiation and external hyperthermia in locally advanced, hormone-refractory or radiation recurrent prostate cancer: a preliminary report. Int J Radiat Oncol Biol Phys 2003; 57: 654–64. doi: 10.1016/S0360-3016(03)00625-4 [DOI] [PubMed] [Google Scholar]
- 25.Arcangeli S, Gambardella P, Agolli L, Monaco A, Dognini J, Regine G, et al. . Stereotactic body radiation therapy salvage reirradiation of radiorecurrent prostatic carcinoma relapsed in the prostatic bed. Tumori 2015; 101: e57–e59. doi: 10.5301/tj.5000251 [DOI] [PubMed] [Google Scholar]
- 26.Mbeutcha A, Chauveinc L, Bondiau PY, Chand ME, Durand M, Chevallier D, et al. . Salvage prostate re-irradiation using high-dose-rate brachytherapy or focal stereotactic body radiotherapy for local recurrence after definitive radiation therapy. Radiat Oncol 2017; 12: 49. doi: 10.1186/s13014-017-0789-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee SH, Jung J, Chang SG. Salvage helical tomotherapy for prostate cancer recurrence following definitive external beam radiotherapy: A case report. Oncol Lett 2015; 10: 1044–6. doi: 10.3892/ol.2015.3346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jereczek-Fossa BA, Kowalczyk A, D'Onofrio A, Catalano G, Garibaldi C, Boboc G, et al. . Three-dimensional conformal or stereotactic reirradiation of recurrent, metastatic or new primary tumors. Analysis of 108 patients. Strahlenther Onkol 2008; 184: 36–40. doi: 10.1007/s00066-008-1783-9 [DOI] [PubMed] [Google Scholar]
- 29.Jereczek-Fossa BA, Bossi-Zanetti I, Mauro R, Beltramo G, Fariselli L, Bianchi LC, et al. . CyberKnife robotic image-guided stereotactic radiotherapy for oligometastic cancer : A prospective evaluation of 95 patients/118 lesions. Strahlenther Onkol 2013; 189: 448–55. doi: 10.1007/s00066-013-0345-y [DOI] [PubMed] [Google Scholar]
- 30.Orecchia R, Surgo A, Muto M, Ferrari A, Piperno G, Girardi MA, et al. . VERO® radiotherapy for low burden cancer: 789 patients with 957 lesions. Ecancermedicalscience 2016; 10: 377. doi: 10.3332/ecancer.2016.677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jereczek-Fossa BA, Piperno G, Ronchi S, Catalano G, Fodor C, Cambria R, et al. . Linac-based stereotactic body radiotherapy for oligometastatic patients with single abdominal lymph node recurrent cancer. Am J Clin Oncol 2014; 37: 227–33. doi: 10.1097/COC.0b013e3182610878 [DOI] [PubMed] [Google Scholar]
- 32.Jereczek-Fossa BA, Fariselli L, Beltramo G, Catalano G, Serafini F, Garibaldi C, et al. . Linac-based or robotic image-guided stereotactic radiotherapy for isolated lymph node recurrent prostate cancer. Radiother Oncol 2009; 93: 14–17. doi: 10.1016/j.radonc.2009.04.001 [DOI] [PubMed] [Google Scholar]
- 33.Jereczek-Fossa BA, Fanetti G, Fodor C, Ciardo D, Santoro L, Francia CM, et al. . Salvage Stereotactic Body Radiotherapy for Isolated Lymph Node Recurrent Prostate Cancer: Single Institution Series of 94 Consecutive Patients and 124 Lymph Nodes. Clin Genitourin Cancer 2017; 15: e623–e632. doi: 10.1016/j.clgc.2017.01.004 [DOI] [PubMed] [Google Scholar]
- 34.Jereczek-Fossa BA, Beltramo G, Fariselli L, Fodor C, Santoro L, Vavassori A, et al. . Robotic image-guided stereotactic radiotherapy, for isolated recurrent primary, lymph node or metastatic prostate cancer. Int J Radiat Oncol Biol Phys 2012; 82: 889–97. doi: 10.1016/j.ijrobp.2010.11.031 [DOI] [PubMed] [Google Scholar]
- 35.Roach M, Hanks G, Thames H, Schellhammer P, Shipley WU, Sokol GH, et al. . Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys 2006; 65: 965–74. doi: 10.1016/j.ijrobp.2006.04.029 [DOI] [PubMed] [Google Scholar]
- 36.Marvaso G, Jereczek-Fossa BA, Riva G, Bassi C, Fodor C, Ciardo D, et al. . High-Risk prostate cancer and radiotherapy: The past and the future. A benchmark for a New Mixed beam radiotherapy approach. Clin Genitourin Cancer 2017; 15: 376–83. doi: 10.1016/j.clgc.2017.01.007 [DOI] [PubMed] [Google Scholar]
- 37.Barchetti F, Panebianco V. Multiparametric MRI for recurrent prostate cancer post radical prostatectomy and postradiation therapy. Biomed Res Int 2014; 2014: 316272–23. doi: 10.1155/2014/316272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kawanaka Y, Kitajima K, Yamamoto S, Nakanishi Y, Yamada Y, Hashimoto T, et al. . Comparison of 11C-choline positron emission tomography/computed tomography (PET/CT) and conventional imaging for detection of recurrent prostate cancer. Cureus 2018; 10: e2966. doi: 10.7759/cureus.2966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pinkawa M, Eble MJ, Mottaghy FM. PET and PET/CT in radiation treatment planning for prostate cancer. Expert Rev Anticancer Ther 2011; 11: 1035–41. doi: 10.1586/era.11.51 [DOI] [PubMed] [Google Scholar]
- 40.Lieng H, Hayden AJ, Christie DRH, Davis BJ, Eade TN, Emmett L, et al. . Radiotherapy for recurrent prostate cancer: 2018 Recommendations of the Australian and New Zealand radiation oncology genito-urinary group. Radiother Oncol 2018;. : : S0167-8140(18)33342–5. doi: 10.1016/j.radonc.2018.06.027 [DOI] [PubMed] [Google Scholar]
- 41.Cheng L, Cheville JC, Bostwick DG. Diagnosis of prostate cancer in needle biopsies after radiation therapy. Am J Surg Pathol 1999; 23: 1173–83. doi: 10.1097/00000478-199910000-00002 [DOI] [PubMed] [Google Scholar]
- 42.Marubini E, Valsecchi MG. Analysing survival data from clinical trials and observational studies. Chichester: The British Institute of Radiology.; 1995. [Google Scholar]
- 43.Kaplan EL, Meier P. Nonparametric Estimation from Incomplete Observations. J Am Stat Assoc 1958; 53: 457–81. doi: 10.1080/01621459.1958.10501452 [DOI] [Google Scholar]
- 44.Volpe S. Salvage image-guided stereotactic second re-irradiation of locally recurrent prostate cancer: something ventured, could something be gained? Congresso Nazionale AIRO – AIRB – AIRO Giovani - Rimini 2016. [Google Scholar]
- 45.Matei DV, Ferro M, Jereczek-Fossa BA, Renne G, Crisan N, Bottero D, et al. . Salvage radical prostatectomy after external beam radiation therapy: a systematic review of current approaches. Urol Int 2015; 94: 373–82. doi: 10.1159/000371893 [DOI] [PubMed] [Google Scholar]
- 46.Zargar H, Lamb AD, Rocco B, Porpiglia F, Liatsikos E, Davis J, et al. . Salvage robotic prostatectomy for radio recurrent prostate cancer: technical challenges and outcome analysis. Minerva Urol Nefrol 2017; 69: 26–37. doi: 10.23736/S0393-2249.16.02797-1 [DOI] [PubMed] [Google Scholar]
- 47.Dipasquale G, Zilli T, Fiorino C, Rouzaud M, Miralbell R. Salvage reirradiation for local failure of prostate cancer after curative radiation therapy: Association of rectal toxicity with dose distribution and normal-tissue complication probability models. Adv Radiat Oncol 2018; 3: 673–81. doi: 10.1016/j.adro.2018.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carrie C, Hasbini A, de Laroche G, Richaud P, Guerif S, Latorzeff I, et al. . Salvage radiotherapy with or without short-term hormone therapy for rising prostate-specific antigen concentration after radical prostatectomy (GETUG-AFU 16): a randomised, multicentre, open-label phase 3 trial. Lancet Oncol 2016; 17: 747–56. doi: 10.1016/S1470-2045(16)00111-X [DOI] [PubMed] [Google Scholar]
- 49.Shipley WU, Seiferheld W, Lukka HR, Major PP, Heney NM, Grignon DJ, et al. . Radiation with or without antiandrogen therapy in recurrent prostate cancer. N Engl J Med 2017; 376: 417–28. doi: 10.1056/NEJMoa1607529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Golbari NM, Katz AE. Salvage Therapy Options for Local Prostate Cancer Recurrence After Primary Radiotherapy: a Literature Review. Curr Urol Rep 2017; 18: 63. doi: 10.1007/s11934-017-0709-4 [DOI] [PubMed] [Google Scholar]