Introduction
Takotsubo cardiomyopathy (TCMP), also known as stress cardiomyopathy or broken‐heart syndrome, is an increasingly recognized form of transient left ventricular (LV) dysfunction that is often completely reversible.1, 2, 3, 4 It is diagnosed in ≈1% to 2% of patients initially presenting with symptoms suggestive of acute coronary syndrome.2 It often presents with dyspnea, hypotension, syncope, elevated troponin levels, and ST elevations or T wave inversions on electrocardiography.1 Coronary angiography classically reveals normal coronary arteries with no significant stenosis, though bystander coronary disease could be present, which is often mild and not severe enough to account for the degree of ventricular dysfunction.1, 2 Complications can occur early in its course and include arrhythmia, thrombus formation, LV outlet tract obstruction, ventricular rupture, cardiogenic shock, and cardiac arrest.1, 3 An up to 8% mortality rate has been reported with excellent recovery in 95%.3 Classically it has been described following a physical and emotional event, with recurrence rates of 10% to 11% on revival of triggering factors.1
Several variants of TCMP have been described. The typical or apical variant consists of a hyperkinetic LV base with focal apical akinesis resulting in apical ballooning and reduced ejection fraction. Other variants such as the inverted or basal pattern (circumferential basal hypokinesis and apical hypercontractility), the mid LV variant (circumferential midventricular hypokinesis and both basal and apical hypercontractility), and the biventricular apical and right ventricular pattern have been described.1, 4
The exact pathogenesis is not fully understood with vast unanswered questions remaining, though a central role of catecholamines has been widely accepted. The stress related to metabolic dysfunction can be the initial trigger for the sympathetic surge found in this disorder. Several case reports have described its occurrence secondary to a number of endocrine disorders, though this has not been comprehensively evaluated. Here we have attempted to review all of the published reports of associations of endocrine conditions with TCMP and discuss some postulated mechanisms by which this may occur. This review will mainly focus on the potential association of pituitary, thyroid, adrenal, and estrogen metabolic disorders with this transient reversible cardiomyopathy and how hormones can impact cardiac function. This review suggests that hormonal dysregulation should be considered in patients presenting with cardiac abnormalities.
Pathophysiology of TCMP
The pathophysiology of TCMP is complex and numerous mechanisms have been postulated, including hypothalamic–pituitary–adrenal axis‐mediated sudden sympathetic activation and surge in concentrations of circulating catecholamines, neuroendocrine circuit, multivessel coronary vasospasm, coronary thrombus, and plaque rupture in the left anterior descending artery wrapped around the apex with rapid autolysis of the clot.5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15 Reversible perfusion defects, sympathetically mediated microvascular dysfunction, and coronary vasospasm have been documented in patients with TCMP.16, 17, 18 However, these observational findings were not supported in a rat model of takotsubo‐like cardiac dysfunction.16 Nevertheless, catecholamine‐driven cardiac dysfunction remains the predominant hypothesis (Figure 1).
Figure 1.
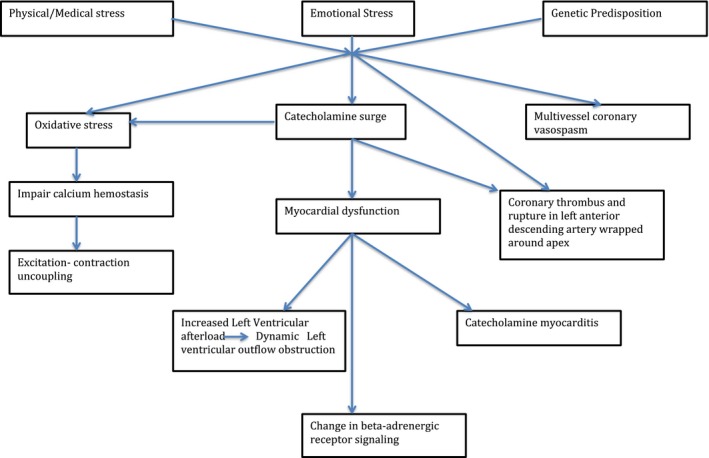
Pathophysiology of takotsubo cardiomyopathy.
The pathophysiology can be broadly divided in 2 phases. The first phase starts with cognitive centers of the brain with a special role of the hypothalamic–pituitary–adrenal axis resulting in appropriate catecholamine release in response to stress. At the onset, serum catecholamine and plasma brain natriuretic peptide levels are elevated significantly in TCMP, even higher than the levels found in patients with acute cardiac decompensation secondary to an ischemic event, supporting a role of hypothalamic–pituitary–adrenal gain and a sympathetic‐driven pathogenesis. Serum catecholamine levels subsequently decline but remain substantially higher than in those patients with acute myocardial infarction after 1 week of onset. In contrast, plasma brain natriuretic peptide levels decline rapidly in TCMP compared with acute myocardial infarction correlating with rapidly improving LV systolic function.
The second phase is the cardiac response to released catecholamines. Transient myocardial stunning often results from direct cardiomyocyte toxicity via increased calcium, microvascular dysfunction or vascular spasm, and ischemia secondary to excess catecholamines.5, 6, 7 Increased sympathetic and adrenomedullary outflow can also cause increased LV afterload leading to outflow tract obstruction, catecholamine myocarditis, and changes in β‐adrenergic receptor signaling.8 Oxidative stress often exaggerates myocardial stunning by affecting calcium homeostasis and causing excitation‐contractive uncoupling resulting in myocardial dysfunction.6, 7, 8 The histopathology of the myocardium in this condition differs from that found in ischemic insults and includes a neutrophil‐predominant inflammation, contraction band necrosis, and fibrosis.9 These changes probably reflect calcium cardiotoxicity rather than ischemic necrosis.
The pattern of ventricular wall abnormality in TCMP also points towards a neurally mediated mechanism of cardiac injury.11, 12, 13 Certain variants of TCMP can be attributed to involvement of different branches of the cardiac sympathetic system, though it has not been fully mapped yet.13 In the classical variant, apical ballooning and akinesis are associated with a hyperkinetic basal segment. A number of anatomic and physiologic factors have been thought to make the apical region of the heart more vulnerable to wall abnormality. Anatomically, it is located in a watershed area of major branches of coronaries arteries, making it more susceptible to developing ischemia. Structurally, the apex lacks a 3‐layered myocardium and ability to regain elasticity after maximal expansion. Physiologically, there are increased sympathetic terminals in the apex as compared with the base, which makes it more susceptible to sudden catecholamine surges. However, in other atypical forms of TCMP, the apex may actually be hypercontractile.
Various theories have been postulated including a migratory form in which apical hypercontractibility may occur as part of the spectrum.13 Rupture of atherosclerotic plaque does not explain the differential involvement itself, though a reduction in the number of synaptic terminals at the apex after akinesis has been suggested as the apex actually recovers fairly quickly after global dysfunction.11, 12, 13, 14, 15 Excessive LV afterload has also been studied as an underlying mechanism.19, 20, 21 Normally, there is uniform distribution of wall stress along the left ventricle (LV). However, if regional wall stress develops quickly, the LV may not have time to adapt and this may result in regional dysfunction. Recently, increased LV end‐systolic pressure has been documented in the acute phase.19 It is postulated that this is exaggerated in elderly women, as on average they have lower mass and LV volume than men and with increasing age adaptation is further limited.20 Interestingly, LV outflow obstruction is dynamic and may not be routinely observed at the time of echocardiographic examination. A genetic predisposition to TCMP has been suggested. Recurrence of TCMP in 1 person and familial associations point towards a potential role of genetic factors. For example, polymorphisms in adrenergic receptors and FMR 1 gene mutations have been reported.22, 23 However, expression profiling of cardiac genes in the acute phase of TCMP has not been complete and remains a subject of ongoing research.
Endocrine Dysregulation and Association With TCMP
Pheochromocytoma and TCMP
Pheochromocytoma is a rare neuroendocrine tumor that usually originates from the adrenal gland chromaffin cells or extra‐adrenal paraganglions, resulting in overproduction of catecholamines.24 Since the sympathetic system still remains the cornerstone for TCMP pathogenesis with special emphasis on β‐adrenergic receptor trafficking in the presence of excess catecholamines, an association of these 2 conditions seems plausible. Many of the diagnostic guidelines exclude pheochromocytoma before diagnosing a patient with TCMP, while others recognize the occurrence of TCMP‐like myocardial dysfunction in these patients.25, 26
Catecholamine‐associated cardiac complications are associated with increased morbidity and mortality.24, 27 At physiological levels, epinephrine is primarily inotropic through β1 and β2 adrenergic receptors. However, at supraphysiological levels, stimulation of β1 receptors produces cardiomyocyte apoptosis and stimulation of β2 receptors results in negative inotropic effects, possibly from a switch in β2 receptor coupling.27 Myocardial alteration and cardiomyopathy secondary to sympathetic stimulation are often reversible with pharmacological and surgical treatment of the pheochromocytoma. However, recently it has been suggested that subclinical systolic and diastolic dysfunction may persist over time despite cure of the underlying pathology with no biochemical evidence of ongoing catecholamine activity.
Agrawal et al reported the prevalence of “idiopathic” pheochromocytoma‐related nonischemic cardiomyopathy to be 17% in their overall cohort, and 25% in patients who underwent diagnostic cardiac imaging, findings that are slightly higher than 11% reported previously.28, 29, 30 Coupez et al have reported 7.5% of TCMP patients to be subsequently diagnosed with pheochromocytoma.31 A review of cases with pheochromocytoma and TCMP by Y‐Hassan et al32 showed the mean age of presentation to be 19.8 years earlier than the usual presentation, with the majority being female. In all 80 cases, pheochromocytoma was cited as the triggering factor, though 24.7% of cases had additional triggers as well. In 76.25% of cases, additional symptoms suggestive of pheochromocytoma apart from chest pain (dizziness, pallor, palpitations, profuse sweating, headache, and hypertension) were present. The regional pattern reported was apical in 43.75%, basal in 30%, midventricular in 5%, global in 20%, and focal in 1.25%. Combined in‐hospital complications were documented in 67.9% of cases. Maximum complications were associated with global involvement followed by basal and apical patterns. The results were similar to those reported in previous case series.33
The diagnosis of pheochromocytoma is often missed on the initial presentation of patients diagnosed with TCMP.24, 32, 33 It is often later that the diagnosis is made after heightened suspicion of excessive secretion of vasoactive peptides, nonresolving symptoms, and inability to attain hemodynamic stability prompt a search for an alternative cause. Pheochromocytoma is often associated with atypical presenting symptoms, a history of malignant hypertension or unstable hemodynamics on presentation.32, 33 Furthermore, TCMP precipitated by a pheochromocytoma is associated with higher rates of in‐hospital complications such as noncardiogenic pulmonary edema, cardiogenic shock, and heart failure.32, 33 Recognition of pheochromocytoma is critical, particularly in association with TCMP, as routine administration of a β‐blocker could be hazardous in the setting of unopposed hyperactivation of α‐adrenergic receptors, further worsening the condition. Therefore, high suspicion is warranted in the clinical setting because both of the processes are catecholamine driven. The underlying factors for catecholamine excess may be multifactorial as well. Screening for pheochromocytoma should always be considered in the diagnostic workup for TCMP, especially in the younger population, when the presentation has atypical features, and when no trigger can be identified.
As mentioned above, paragangliomas are also neuroendocrine tumors associated with catecholamine excess, and hence it is plausible that catecholamine‐mediated cardiomyopathy such as TCMP could be observed in this setting as well. Gagnon et al reported an incidence of acute takotsubo‐like cardiomyopathy of 2.6% in a series of 152 patients with catecholamine‐secreting paragangliomas.34 Giavarini et al evaluated 140 consecutive patients with paragangliomas and found episodes of acute catecholamine cardiomyopathy in 15 (11%) of them, with 6 having features of TCMP.29
Menopause and TCMP
TCMP has primarily been described as a disease affecting elderly postmenopausal women with a 6‐fold female–male predominance, leading researchers to postulate an estrogen‐based theory for the pathogenesis of this reversible transient cardiomyopathy.35, 36, 37, 38, 39, 40, 41 Sy et al reported an incidence of TCMP of 5.9% in a cohort of 1297 postmenopausal women who presented with elevated troponin levels.42 Estrogen modulates vascular tone by upregulating endothelial nitric oxide synthase activity and inhibiting endothelial apoptosis in response to vascular injury. Although estrogen can increase circulating norepinephrine levels, which can result in vasoconstriction, there is net vasodilation in response to increased activity of endothelial nitric oxide synthase. Moreover, women are less sensitive to the hypertensive effects of the renin–angiotensin system as a result of upregulation of type 2 angiotensin II receptors.39 Estrogen receptors are also expressed in cardiac tissue and can play a role in modulating catecholamine effects on the heart.
Experiments on rat heart have shown cardioprotective effects of estrogen via blunting of catecholamine‐induced tachycardia and reducing ischemia/reperfusion–induced arrhythmias.39, 40 Ueyama et al demonstrated upregulation of cardioprotective peptides and attenuation of sympatho‐adrenal outflow after chronic estrogen supplementation in an animal model exposed to immobilization stress.38, 39 Another study demonstrated reversal of upregulation of β1 receptors and increased cardiac sensitivity in bilateral ovariectomized rats after estrogen supplementation.40 Kuo et al described a single‐center review of TCMP in postmenopausal women, none of whom were taking estrogen replacement therapy.35 Another study by Komesaroff et al demonstrated attenuated catecholamine and glucocorticoid response to mental stress in perimenopausal women after estrogen supplementation.43 Estrogen deficiency in the menopausal state can result in exaggerated reactivity to stress via an autonomic surge in the absence of cardioprotective peptides (Figure 2). A role of estrogen supplementation in postmenopausal women to protect against TCMP has been suggested, but human trial data in this regard are scant.
Figure 2.
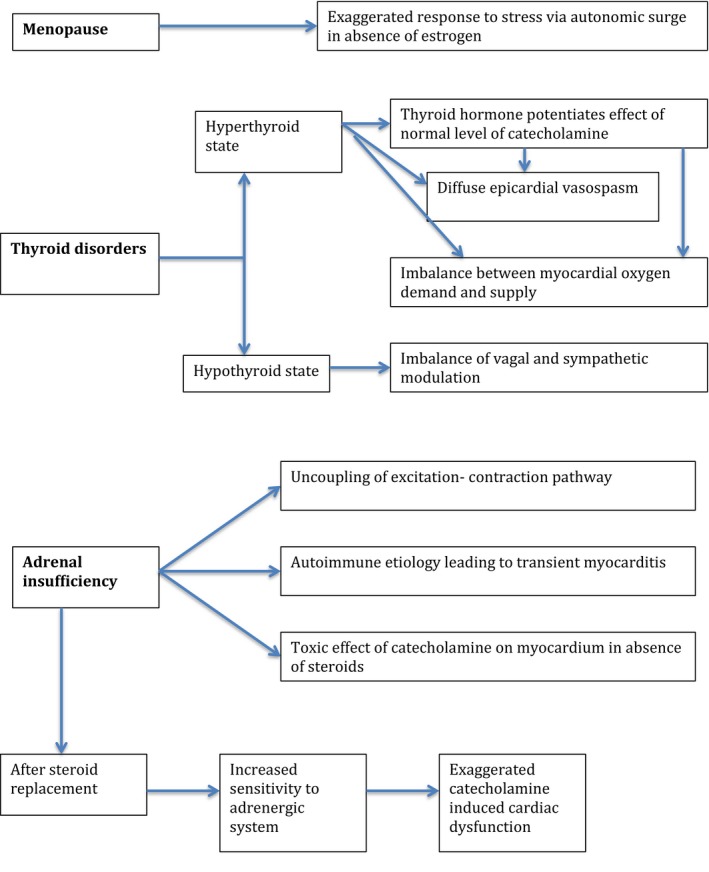
Pathophysiology of takotsubo cardiomyopathy in relation to underlying endocrine conditions.
Thyroid Disorders and TCMP
Recently there has been increasing evidence to suggest the occurrence of TCMP in patients with thyroid dysfunction. It has been reported to occur in patients with Graves disease, Hashimoto thyroiditis, toxic multinodular goiter, apathetic hyperthyroidism, thyroid storm, iatrogenic hyperthyroidism, subclinical hyperthyroidism, transient hyperthyroid states, following radioactive iodine treatment, following thyroidectomy, and even in hypothyroid or euthyroid states.44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71 However, the mechanism by which different thyroid functional states can trigger TCMP remains unclear. Table 1 summarizes case reports describing TCMP occurring in patients with thyroid disorders.
Table 1.
Case Reports of TCMP With Thyroid Disorders
| Authors (Year)Reference | Age/Sex | Endocrine Disorder | Presentation | Cardiac Enzymes | ECG | TTE | Cardiac Catheterization/Left Ventriculogram | Recovery of Cardiac Function |
|---|---|---|---|---|---|---|---|---|
| Gowda et al (2003)71 | 51 yo/F | Iatrogenic hyperthyroidism | Chest pain, dyspnea | Elevated | Sinus rhythm with diffuse ST‐T abnormalities | Mild LV systolic dysfunction with global hypokinesis | Normal coronaries, LV EF 48%, diffuse hypokinesis | 4‐mo follow‐up: asymptomatic |
| Miyazaki et al (2004)56 | 79 yo/F | Grave disease | Palpitation | Normal | Elevated ST segment and reduction of R wave voltage in the right precordial leads | Akinetic motion of the anteroseptal wall, apex, and inferior wall, EF 45% | Normal coronaries, apical akinesis, EF 45% | Day 9: recovery of LV function |
| Sakaki et al (2004)47 | 74 yo/F | Transient hyperthyroidism (Hashimoto thyroiditis) | Chest discomfort | Elevated | ST elevation in leads II, III, aVF, V3 to V6 | Hypokinesis left ventricular apex | Normal coronaries, | 2‐wk follow‐up: apical dysfunction resolved |
| Rossor et al (2007)55 | 61 yo/F | Hyperthyroidism secondary to Grave disease, trigger exercise induced | Dyspnea | Elevated | Anterolateral T wave inversion | Focal apical akinesia | Normal coronaries, LV dysfunction | Follow‐up: recovery of LV dysfunction |
| Sarullo et al (2009)52 | 55 yo/F | Thyrotoxicosis secondary to Grave disease | Dyspnea, pulmonary edema | Elevated | Sinus tachycardia with ST‐elevation in leads D1, aVL and V1 to V4 | Apical akinesis, EF 28% | Normal coronaries | Day 18: recovery of LV function |
| Radhakrishnan et al (2009)54 | 65 yo/F | Grave disease | Weight loss, diarrhea, dyspnea | Elevated | Sinus tachycardia, ST elevation V1 to V3 | Akinesis apical and mid‐ventricular segments, LV cavity dilated, EF 25% | Normal coronaries | Day 4: EF 60%, resolution of apical wall abnormalities |
| Bilan et al (2009)45 | 59 yo/F | Hyperthyroidism secondary to Grave disease, COPD | Dyspnea at rest | Normal | Sinus rhythm, ventricular ectopic beats, small ST‐segment elevation of 0.5 mm in the precordial leads V4 to V6 | Apical and mid‐ventricular akinesis, hyperkinesis of left ventricular parabasal segments, EF 35% | Normal coronaries | 1 wk later: EF 50% |
| Van de Donk et al (2009)46 | 73 yo/M | Recurrent hyperthyroidism (toxic multinodular goiter) treated with radioactive iodine | Dyspnea | Elevated | Sinus tachycardia with ST‐segment elevation in the anterior precordial leads and T‐wave inversion in the lateral leads | Apical akinesia and hyperkinetic base, EF 25% | Normal coronaries, apical ballooning, LV EF25% | 7‐wk follow‐up: EF 65% |
| Tsao et al (2010)49 | 31 yo/F | Chinese herb nephropathy, thyrotoxicosis factitia | Chest pain | NA | NA | NA | Normal coronaries, apical ballooning | 2‐wk follow‐up: complete recover |
| Alidajan et al (2010)53 | 66 yo/F | Grave disease | Nausea, vomiting, palpitation | Elevated | Sinus tachycardia | EF 40%, LV end diastolic dimension and wall thickness normal | Normal coronaries, apical ballooning, EF 40% | 1‐mo follow‐up: normal EF |
| Kwon et al (2010)50 | 55 yo/F | Iatrogenic thyrotoxicosis | Chest pain, seizure | Elevated | Sinus tachycardia, no ST changes | Depressed LV systolic function, akinesia of all apical and midventricular segments with sparing of the basal segments | Normal coronaries, apical akinesis | 3‐mo follow‐up: recovery of LV function |
| Hutchings et al (2010)48 | 79 yo/F | Untreated hyperthyroid | Chest pain, breathlessness, anxiety | Elevated | Anterior T‐wave inversion | NA | Nonobstructive coronaries, apical ballooning, LV dysfunction | Day 5: recovery of LV function |
| Hutchings et al (2010)48 | 55 yo/F | Iatrogenic porcine thyroxine intake | Dyspnea, chest pain, cardiogenic shock, pulmonary edema | Elevated | Anterolateral T‐wave inversion | NA | Mild atherosclerosis, apical akinesis, basal hyperkinesia | 4‐mo follow‐up: complete recovery |
| Kuboyama et al (2011)59 | 84 y/o F | Thyrotoxicosis | Palpitations | Elevated | Inversion of T waves at V3 to V6 | Depressed LV systolic function around apex region | Normal coronaries, apical akinesis, midbasal hyperkinesia | Day 5: improved LV function |
| Dahdouh et al (2011)61 | 53 yo/F | Thyrotoxicosis | Chest pain | Elevated | Sinus rhythm with infero‐apico‐lateral ST‐segment elevation | Severe LV systolic dysfunction, EF 30% with large apical ballooning | Nonobstructive atherosclerosis, apical ballooning, EF 30% | Day 20: recovery of LV function |
| Day 30: chest pain, weight loss, diarrhea | NA | Diffuse deep T waves inversion with prolonged corrected QT interval | Apical ballooning | NA | 2‐mo follow‐up: LV recovery | |||
| Micallef et al (2011)60 | 58 yo/F | Primary hypothyroid secondary to radioiodine therapy | Lethargy, dyspnea | Elevated | Deep T‐wave inversions in V2 to V6, leads II and aVF | Global hypokinesis except base | Normal coronaries, apical akinesis | 4‐wk follow‐up: recovery EF 55% |
| Zuhdi et al (2011)65 | 82 yo/F | Thyrotoxicosis | Chest pain, dyspnea, palpitation, diaphoresis | Elevated | Sinus tachycardia with ST‐segment elevation in the inferolateral leads | Global akinesis except basal part of LV wall, EF 18% | Mild obstructive coronary, apical ballooning | 5‐mo follow‐up: normal EF |
| Gundara et al (2012)51 | 40 yo/F | After thyroidectomy in Grave disease | Anxiety, dyspnea, chest pain | Elevated | Sinus tachycardia with inverted T waves in V1 to V2 | Ventriculogram: moderate diffuse LV apical hypokinesis and ballooning | Normal coronaries, LV dysfunction with apical akinesis | Follow‐up: recovery of LV function |
| Hatzakorzian et al (2013)63 | 68 yo/F | Obstructive goiter | Dyspnea, fatigue | Slightly elevated | Atrial fibrillation, rate 170/min | EF 30% with severe apical hypokinesis | Normal coronaries, apical hypokinesis, EF 30% | 1‐wk follow‐up: EF recovered |
| Wu et al (2014)62 | 81 yo/F | Diabetic ketoacidosis, thyroid storm, Grave disease | Palpitation, chest tightness, abdominal fullness, vomiting, diarrhea | Elevated | Sinus tachycardia with ST elevation over V2 to V4 | Impaired LV systolic function (EF: 35.4%) with apical hypokinesia to akinesia | Normal coronaries, apical hypokinesis akinesis, LV systolic dysfunction, EF 35% | Day 3: EF 59% |
| Perkins et al (2014)57 | 36 yo/F | Hyperthyroidism secondary to Grave disease | Epigastric pain, nausea, vomiting, diarrhea, weight loss | Normal | New T‐wave inversions in the precordial leads | Left and right ventricular apical akinesis, EF 25% | Normal coronaries, apical akinesis, hyperkinetic base | 6‐wk follow‐up: normal LV function |
| Eliades et al (2014)44 | 71 yo/F | Treated diabetes ketoacidosis, thyrotoxicosis | Abdominal pain, vomiting, confusion, weight loss | Elevated | ST and T‐wave changes consistent with anterolateral ischemia | NA | Normal coronaries, apical midcavitary hypokinesis, EF 30% | Day 10: EF 45%–50% |
| Martin et al (2014)58 | 47 yo/F | Non autoimmune destructive thyroiditis | Chest pain, dyspnea, palpitation, diaphoresis | Elevated | ST segment elevation, negative T waves | Apical akinesis, EF 35% | Normal coronaries, apical ballooning, EF 35% | Few wks later: full recovery |
| Al‐Salameh et al (2014)68 | 70 yo/F | Apathetic hyperthyroidism | Chest pain | Elevated | ST segment elevation in apical and lateral leads | Large apical dyskinetic region, reduced EF | Normal coronaries, apical akinesis | Follow‐up: recovery of LV function |
| Omar et al (2015)70 | 61 yo/F | Thyrotoxicosis secondary to Grave disease | Dyspnea, palpitation | Elevated | Atrial fibrillation with rapid ventricular response and nonspecific ST‐T‐wave changes | EF 35%–39% and akinesis of septal and apical region | Normal coronaries, apical akinesis and ballooning. EF 35%– 39% | 3‐mo follow‐up: EF 60% |
| Patel et al (2016)67 | 55 yo/F | Grave disease | Chest pain, dyspnea | Elevated | Premature ventricular beats and nonspecific ST changes in the anteroseptal leads | EF 30%, akinesis of mid‐to‐distal anterior, lateral, inferior, septal walls and apex | Diffuse luminal irregularities with all stenosis <30% in severity, apical ballooning, EF 30% | Follow‐up 2 mo: EF 75% |
| 20 d after stopping methimazole: dyspnea | Elevated | New deep, symmetric T‐wave inversions | EF 40% with akinesis of apex and distal anterior, lateral, inferior, and septal walls | Unchanged nonobstructive coronary artery disease, akinesis apex, EF 40% | Follow‐up 9 mo: EF recovered to 55% | |||
| Brenes‐Salazar et al (2016)64 | 65 yo/F | Primary hypothyroidism | Fatigue, weakness, lightheadedness, chest pain | Elevated | Normal sinus rhythm with new T‐wave inversions in anterolateral leads | NA | Normal coronaries, apical akinesis, basal hyper contractility | Day 4: improved symptoms |
| Murdoch et al (2016)69 | 67 yo/F | Hyperthyroidism, multinodular goiter | Respiratory distress, palpitation | Elevated | Sinus tachycardia without ST changes | Akinesis of the lateral, anterior, and septal apex and well‐preserved basal function, with EF 40%–45% | Non‐obstructive coronaries. | 6 weeks follow up: EF 72% |
| Rueda et al (2017)66 | 34 yo/M | Hyperthyroidism secondary to Grave disease | Chest pain, dyspnea | Elevated | T‐wave inversion in DIII, aVF, V5 to V6 | Severe apical and moderate anterior hypokinesis and EF 40% | Normal coronaries, apical akinesis, EF 40% | 6‐wk follow‐up: LV EF recovered |
aVF indicates Augmented Vector Foot; aVL, Augmented Vector Left; COPD, ; EF, ejection fraction; LV, left ventricle; NA, not applicable; TCMP, takotsubo cardiomyopathy; TTE, transthoracic echocardiogram.
Thyroid hormone effects on the cardiovascular system are well documented and hyperthyroid states can result in increased cardiac output, up to 300% higher than that found in euthyroid individuals.70, 71, 72, 73, 74 Thyroid hormone increases heart rate and myocardial contractility while simultaneously decreasing systemic vascular resistance, thereby resulting in activation of the renin–angiotensin system. Both genomic and nongenomic actions have been suggested. Triiodothyronine, the active form of thyroid hormone, acts at the nuclear level and increases transcriptional activation of cardiac proteins, both structural and regulatory proteins such as Na+/K+ ATPase, sarcoplasmic reticulum Ca2+‐ATPase, voltage‐gated ion channels, myosin heavy chain and β‐adrenergic receptors. Nongenomic effects involve membrane ion channels and endothelial nitric oxide synthase, resulting in vascular relaxation and decreased systemic resistance.72, 73
The thyroid and adrenergic systems are closely linked, and hyperthyroid states indeed resemble hyperadrenergic states. It is suggested that thyroid hormones potentiate the actions of normal levels of catecholamines and result in exaggerated inotropic and chronotropic effects (Figure 2). Findings of upregulation of β‐adrenergic receptors in the heart support this notion, although this is not always accompanied by simultaneous cardiac sensitivity to catecholamines.74 Some studies have documented low‐to‐normal catecholamine levels in hyperthyroid states.75 Animal studies have shown an intact cardiovascular response to hyperthyroidism even when all 3 β‐adrenergic receptors were blocked.76 This raises the possibility of a mechanism other than an adrenergic‐mediated one. Some reports suggest that epicardial arterial spasm may occur, resulting in vasospastic angina, while others have hypothesized a critical imbalance of myocardial oxygen supply and demand exclusive of coronary spasm or coronary artery disease.77, 78, 79
TCMP has also been documented to occur in patients with hypothyroid or subclinical hypothyroid states.60, 80 In a retrospective study, Aggarwal et al found an association of TCMP with thyroid disorders and reported 35% of the patients to be hypothyroid, with the majority of them being on thyroid replacement and 11.36% being in a hyperthyroid state.80 However, it is unclear whether the pathogenesis is solely related to the thyroid functional state or if other confounding variables are involved.79, 80 Studies have reported alterations of the autonomic nervous system in patients with hypothyroidism, resulting in an imbalance of vagal and sympathetic modulation (Figure 2).81, 82, 83 Moreover, the hypothyroid state is known to increase the risk of atherosclerosis and cardiovascular events, increase peripheral resistance, and reduce ventricular function.74 Proper interpretation of thyroid function testing in the context of the clinical picture is important because patients with Graves disease can present as hyperthyroid or hypothyroid at different times, depending on the ratio of stimulating and blocking thyroid antibodies, commonly described as the “thyroid yo‐yo.”84, 85 Also, it should be noted that not all patients with Graves disease actually have thyroid antibodies. Moreover, thyroid tracer uptake may be suppressed in patients on treatment, although increased uptake is expected in Graves disease.86
Larger studies are needed to better understand the potential association between thyroid disorders and TCMP. In the interim, physicians should be aware of this potential relationship and consider ordering appropriate thyroid function testing in the relevant clinical setting to facilitate prompt diagnosis and treatment.
Adrenal Insufficiency and TCMP
Adrenal insufficiency is a life‐threatening entity caused by insufficient glucocorticoid production87 and is described as primary, secondary, or tertiary, depending on the cause. Primary adrenal insufficiency results from adrenal cortex diseases such as autoimmune disease, infections, hemorrhage, or rarely, malignancy. Central adrenal insufficiency, including secondary and tertiary adrenal insufficiency, is caused by disorders of the hypothalamic–pituitary axis. Presenting symptoms of adrenal insufficiency are often nonspecific, resulting in delayed diagnosis and treatment. Adrenal insufficiency has been associated with changes in cardiac and vascular function, with patients presenting with volume depletion, electrolyte disturbances, diastolic hypotension, decreased stroke volume, and peripheral vascular resistance, cardiac failure, and cardiac atrophy. Impaired hemodynamics with volume contraction and low cardiac output are common in Addisonian crisis, and recovery of normal cardiovascular status is rapidly achieved with prompt fluid resuscitation and steroid therapy.87
Reversible systolic dysfunction resembling TCMP has been documented in primary and secondary adrenal insufficiency,88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98 although the pathophysiologic mechanism remains unknown. There have been conflicting reports in that while in some patients cardiac dysfunction resolved on steroid replacement, in others it worsened. Glucocorticoids play an important role in myocardial contractility by maintaining calcium transport across the cardiac sarcoplasmic reticulum and modulating the vascular response to β‐agonists and maintaining cardiac inotropy.99, 100, 101, 102, 103, 104
In primary adrenal insufficiency, as found in animal experimental models, there is uncoupling of the excitation–contraction pathway resulting in myocardial injury (Figure 2).100 Studies in animals have suggested a toxic effect of catecholamines on the myocardium in the absence of glucocorticoids, when adrenalectomized animals were exposed to stress.101 However, exposure of cortisone‐starved myocardium to steroid replacement results in greater sensitivity to the adrenergic system, exaggerating catecholamine‐induced cardiac dysfunction, and resulting renal maladaptation leading to excessive water and sodium retention as well as a direct toxic effect of steroids on chronically steroid‐deprived tissues.102, 103 It is unknown whether underlying ischemic, valvular, and hypertensive heart disease in the elderly population is a predisposing factor because the cases describing worsening cardiac function after replacement therapy did not have underlying cardiac disease. Internists, cardiologists, intensivists, and endocrinologists should be aware of this complex association between adrenal insufficiency and cardiac dysfunction, which can often worsen on steroid replacement therapy. It is difficult to estimate the prevalence of TCMP in patients with adrenal disorders, because current information is based on case reports. Details of the 12 cases reporting such an association are summarized in Table 2.
Table 2.
Case Reports of TCMP and Adrenal Disorders
| Authors (Year)Reference | Age/Sex | Endocrine Disorder | Presentation | Cardiac Enzymes | ECG | TTE | Cardiac Catheterization/Left Ventriculogram | Recovery of Cardiac Dysfunction |
|---|---|---|---|---|---|---|---|---|
| Iga et al (1992)88 | 64 yo/F | Secondary adrenal insufficiency, low ACTH, low cortisol | Infection | Normal | Negative deep T waves in precordial leads | LV systolic dysfunction | Normal coronaries, apical akinesis | Day 5: recovery of LV wall motion |
| 74 yo/F | Postop knee surgery | Normal | Negative deep T waves in precordial leads | |||||
| Eto et al (2000)96 | 62 yo/M | Primary adrenal insufficiency secondary to empty sella | Volume overload | NA | Prolonged QTc interval (0.62 s) and negative T wave on the right precordial leads | EF 37%, LV enlargement | Normal coronaries | 2‐mo follow‐up: EF 52% |
| Oki et al (2006)89 | 74 yo/M | Secondary adrenal insufficiency and hypothyroidism caused by nonfunctioning pituitary adenoma | Coma | Elevated | ST segment elevation in leads V2 to V3 with ST depression in leads II; III, aVF and terminal T inversion in leads V2 to V5 | NA | Normal coronaries, apical akinesis with basal hyperkinesia, EF 45% | Day 14: normal LV wall function |
| Wolff et al (2007)95 | 42 yo/F | Addisonian crisis | Progressive weight loss, nausea, vomiting, and hypotension. Developed cardiorespiratory failure after hydrocortisone therapy | Elevated | Loss of R wave progression in V1 to V4, ST‐segment elevation in V1 to V5, and negative T‐waves in V2 to V6 | Normal ventricular dimensions but severe LV dysfunction with EF 30% | Not done | On discharge: normal wall‐motion and nearly complete recovery of LV function (EF 52%) |
| Sakihara et al (2007)90 | 53 yo/F | Adrenocortical insufficiency caused by isolated ACTH deficiency, chronic thyroiditis, partial empty sella | Loss of consciousness and hypoglycemia (serum glucose: 34 mg/dL) | Elevated | ST elevation and T‐wave inversion in V1 to V6 | NA | Normal coronaries, LV apical ballooning and severe hypokinesis of anterior and posterior walls | Day 14: recovery of LV wall motion and LV EF 70% |
| Gotyo et al (2009)91 | 70 yo/M | Secondary adrenal insufficiency secondary to idiopathic ACTH deficiency | Bacterial pneumonia, cardiopulmonary arrest | Normal | Deep inverted T waves in the chest leads and QT prolongation | LV dysfunction with akinesis of the apex and hyperkinesis of the basal wall | Normal coronaries, akinesis of the LV apex with ballooning during systole | 4‐wk follow‐up: normal LV function and resolution of T waves |
| Ukita et al (2009)92 | 69 yo/F | Acute adrenal crisis, ACTH deficiency | Fatigue, loss of appetite, loss of consciousness, Day 3 developed chest pain | Elevated | Deep negative T waves in leads I, II, III, aVF and V1 to V6 | LV systolic dysfunction, EF 33% | Normal coronaries, ergotamine provocation test nest, akinesis anterolateral, apical, diaphragmatic segments | Day 8: recovery of LV function except apex, EF 74% 3‐wk follow‐up: normal apical function |
| Punnam et al (2010)94 | 71 yo/F | Addisonian crisis | Syncope | Elevated | Subtle ST segment elevations in V2 to V6 leads without any reciprocal changes | EF 25%–30%, dyskinetic apex and inferior wall | Normal coronaries, apical ballooning | 9‐mo follow‐up: normal EF |
| Barcin et al (2010)98 | 40 yo/F | Addison disease | Chest pain | Elevated | Sinus rhythm and negative, symmetrical T waves from V1 to V6 and aVL | EF 44%, apical akinesis, basal hyperkinesis | Normal coronaries, apical akinesis and ballooning | 5‐mo follow‐up: no improvement in apical akinesis |
| Murakami et al (2012)93 | 65 yo/F | Isolated ACTH deficiency | Malaise, nausea, delirium | Elevated | T waves were inverted in leads II, III, V1, and V2 | NA | Normal coronaries, apical akinesis and ballooning | Day 10: recovery of LV wall motion |
| Singh et al (2015)97 | 48 yo/M | Hypopituitarism with secondary adrenal insufficiency in adrenal crisis | Diminished vision, headache, vomiting, altered mental status | Elevated | Sinus tachycardia with T‐wave inversion and ST‐elevation in lead 1, 2, aVF, aVL, V1 to V6, poor R wave progression in V1 to V4 | Regional wall motion abnormality involving LAD territory with EF <40% | Normal coronaries, apical ballooning, EF <30% | 7‐Day follow‐up: EF 52% |
ACTH indicates adrenocortical trophic hormone; aVF, Augmented Vector Foot; aVL, Augmented Vector Left; EF, ejection fraction; LV, left ventricle; NA, not applicable; TCMP, takotsubo cardiomyopathy; TTE, transthoracic echocardiogram.
Diabetes Mellitus and TCMP
Diabetes mellitus with autonomic neuropathy has been associated with a functional deficit in noradrenergic neurons within the hypothalamus and sluggish adrenal epinephrine output, resulting in a low catecholamine state.105, 106, 107 Clinical and animal studies have documented decreased norepinephrine release from local cardiac sympathetic nerves in longstanding diabetes mellitus even with subtle neuropathy. This has been thought to have a protective effect against TCMP via cardiac and splanchnic sympathetic output impairment. However, the association of TCMP in diabetes mellitus remains debatable.
Madias et al described a low prevalence of diabetes mellitus in TCMP in a meta‐analysis after analyzing the global literature on TCMP from 2001 to 2014.108 Another retrospective study by Yayehd et al reported an 11.5% prevalence of diabetes mellitus in a cohort of 117 French patients with TCMP.109 A report based on 2 Spanish cohorts with 328 patients reported the prevalence of diabetes mellitus to be 13.1%.110 These studies have suggested that diabetes mellitus may exert a protective effect against catecholamine‐driven cardiac dysfunction. However, these studies were retrospective and primarily hypothesis‐generating with limited clinical details regarding the duration of diabetes mellitus, the degree of glycemic control, and validation of autonomic neuropathy by clinical testing. In fact, most of the reported literature regarding an association between diabetes mellitus and TCMP does not provide detailed information such as duration of diabetes mellitus, whether it was controlled or uncontrolled, the presence or absence of complications, and the type of diabetes mellitus. The lack of such details can potentially lead to misrepresentation of an association of diabetes mellitus with reversible cardiomyopathy.
Generalizability of the reported findings is further limited in that the overall prevalence of diabetes mellitus in Europe is much lower as compared with the rest of the world.111 However, Templin et al reported a 14.2% prevalence of diabetes mellitus in patients with TCMP enrolled in the International Takotsubo Registry across 9 countries, though on detailed subgroup analysis the prevalence was reduced to 12.8%.112 This low prevalence was significant as compared with a higher prevalence of diabetes mellitus in the general population. Bill et al113 have reported a higher prevalence of diabetes mellitus in TCMP (22.8%) in their cohort as compared with 16.8% reported in a meta‐analysis. This may have been related to a higher overall prevalence of diabetes mellitus in their cohort, but paradoxically the diabetic patients with TCMP had better short‐term and long‐term outcomes.
According to the pathophysiological link between diabetes mellitus and TCMP, longstanding poorly controlled diabetic patients who are exposed to overwhelming stress can develop a reversible cardiomyopathy after counteracting the postulated diabetic TCMP “protective” effect. However, it is difficult to say whether mild glucose impairment exerts the same cardioprotective effect. Diabetes mellitus has been reported to be an independent predictor of mortality in patients with TCMP in 1 study.114 TCMP has also been documented in association with diabetic ketoacidosis, hyperglycemic hyperosmolar states, and poorly controlled diabetes mellitus in case reports.115, 116, 117 Whether stress related to hyperglycemia can be the initial trigger remains unclear. Various explanations postulated include hyperglycemia‐induced coronary microvascular dysfunction along with inflammatory and oxidative stress‐induced cellular dysfunction. Still, many questions remain unanswered and require prospective studies to validate the clinical association.
Hypoglycemia and TCMP
There are a few case reports suggesting hypoglycemia as a trigger for TCMP.118, 119, 120 Most of the cases with hypoglycemia occurred in association with anorexia nervosa or adrenal insufficiency. In others, no cause could be identified. Anorexia nervosa is a complicated entity to assess as regards hypoglycemia, given that it causes several metabolic and electrolyte disturbances and will not be covered further here.121, 122, 123 Table 3 summarizes case reports suggesting hypoglycemia as potential trigger for stress cardiomyopathy. In all 3 cases blood glucose was <40 mg/dL with complete recovery of cardiac function within a few days after correction.
Table 3.
Case Reports of TCMP in Patients With Hypoglycemia
| Authors (Year)Reference | Age/Sex | PMH/Associated Diagnosis | Presentation | BS (mg/dL) | Work‐up | Cardiac Enzymes | ECG | TTE | Cardiac Catheterization/Left Ventriculogram | Recovery |
|---|---|---|---|---|---|---|---|---|---|---|
| Ansari et al (2011)120 | 69 yo/F | None | Unresponsive | 32 | Normal C peptide, proinsulin, sulfonylurea screen negative | Elevated | Sinus tachycardia | Severe LV dysfunction, apical ballooning, EF 16% | Normal coronaries, apical ballooning | Day 5, EF 45% |
| Katoh S et al (2012)118 | 60 yo/F | Type 1 DM, cirrhosis, chronic pancreatitis | Unresponsive | 38 | Negative for pheochromocytoma | Elevated | Prolonged QT interval and tall T waves in leads V3 to V5, II, III, and aVF, without ST‐segment deviation | Basal akinesis/dyskinesia and apical hyperkinesis | Normal coronaries, inverted takotsubo pattern | Day 4, EF 62% |
| Hsu et al (2010)119 | 44 yo/F | Traumatic brain injury, overdose oral hypoglycemic agent | Unresponsive | 8 | NA | Elevated | Sinus tachycardia, ST‐segment depression in leads V4 to V6 | NA | Normal coronaries, basal akinesis/dyskinesia and apical hyperkinesis | Day 9, EF >55% |
aVF indicates Augmented Vector Foot; BS, blood sugar; DM, diabetes mellitus; EF, ejection fraction; LV, left ventricle; NA, not applicable; PMH, past medical history; TCMP, takotsubo cardiomyopathy; TTE, transthoracic echocardiogram.
Hypoglycemia results in compensatory catecholamine secretion, which has been suggested to be the cornerstone of TCMP pathogenesis. The theory of perfusion–metabolism mismatch has been suggested, with diminished apical glucose uptake as demonstrated by positron emission tomography scan with 13N‐ammonia and 18F‐fluorodeoxyglucose.118, 119, 120 Whether hypoglycemic stress results in inverted TCMP more often than classic apical TCMP is yet to be answered, and more data are required before accepting this association.
Autoimmune Polyendocrine Syndrome and TCMP
M. B. Schmidt first described autoimmune polyendocrine syndrome in 1926 in patients with adrenal insufficiency and chronic lymphocytic thyroiditis.124, 125 Three forms of this syndrome have been reported. Type I is a rare disease of childhood with equal sex incidence and consists of mucocutaneous candidiasis, hypoparathyroidism, and primary adrenal insufficiency. It is usually inherited in an autosomal recessive pattern with variable inheritance with no HLA association. Type II is more common in adults, predominantly in women with the main features including primary adrenal insufficiency, autoimmune thyroid disease, and/or type 1 diabetes mellitus. Primary hypogonadism, myasthenia gravis, and celiac disease have also been observed in this syndrome. The pattern of inheritance is often autosomal dominant with variable expressivity and an association with HLA‐DR3 and/or HLA‐DR4 haplotypes. Type III, although ill defined, is the co‐occurrence of autoimmune thyroid disease with organ‐specific autoimmune disorders in the absence of Addison disease.
Case reports have documented an association of cardiac dysfunction with autoimmune polyendocrine syndrome in patients with autoimmune cardiomyopathy, nonreversible dilated cardiomyopathy, peripartum cardiomyopathy, and recently TCMP.126, 127, 128, 129, 130 The exact pathogenesis of transient cardiac dysfunction in these patients with autoimmune polyendocrine syndrome remains unknown, though hormonal preconditioning and sudden hormonal crisis have been suggested as a possibility. In the reported cases by Lim et al and Yehya et al,129, 130 patients were diagnosed with TCMP during adrenal crisis, raising the possibility of an excessive catecholamine surge secondary to hypotension and hypoglycemia playing a central role. Another theory postulated that transient dysfunction of the sodium/calcium ion pump in the setting of hyponatremia, which is often associated with autoimmune polyendocrine syndrome, results in impaired cardiac contractility though only 1 patient presented with severe hyponatremia. Both cases presented with undertreated hypothyroidism, which could itself result in an increased rate of production and prolonged clearance of norepinephrine. Consistent with the literature showing that catecholamine levels are not always elevated in TCMP, in one of these reports catecholamine levels were found to be normal. The 2 cases are summarized in Table 4.
Table 4.
Case Reports of TCMP in Patients With APSII
| Authors (Year)Reference | Age/Sex | Endocrine Disorder | Possible Trigger | Cardiac Enzymes | ECG | TTE | Cardiac Catheterization/Left Ventriculogram | Recovery of Cardiac Dysfunction |
|---|---|---|---|---|---|---|---|---|
| Lim et al (2009)129 | 64 yo/M | APS II (Hashimoto thyroiditis and primary adrenal insufficiency), hypogonadism | Addison crisis: hypotension, hypoglycemia, hyponatremia, dopamine infusion | Elevated |
Sinus rhythm with elongated QT intervals and ST‐segment elevation in leads II, III, aVF, V2 to V6, and I. T wave inversion was seen in all leads |
Severely reduced LV contraction at apical side | Intact coronary arteries and apical ballooning | Hospital Day 6 |
| Yehya et al (2011)130 | 26 yo/F | APSII (Hashimoto thyroiditis, primary adrenal insufficiency) | Addison crisis: hypotension | Elevated | Diffuse ST abnormalities in the anterolateral region | Moderate‐severe reduced LV systolic function. LV ejection fraction (LVEF) 30% | Intact coronaries and apical ballooning. |
Hospital day 3, LVEF 51%. 1 mo later complete recovery |
| Karavelioglu et al (2013)126 | 36 yo/F | APSII (Hashimoto thyroiditis, Addison disease, vitiligo) | Addison crisis, hyperkalemia | Normal | Sinus rhythm with negative T‐wave in precordial and extremity leads | Enlarged LV with a depressed systolic function, akinesis of anterior, apical, mitral, and tricuspid regurgitation | NA | 3‐mo follow‐up: LV function recovered |
APSII indicates Autoimmune polyglandular syndrome type II; aVF, Augmented Vector Foot; EF, ejection fraction; LV, left ventricle; NA, not applicable; TCMP, takotsubo cardiomyopathy; TTE, transthoracic echocardiogram.
Syndrome of Inappropriate Antidiuretic Hormone Secretion and TCMP
Syndrome of inappropriate antidiuretic hormone secretion (SIADH) is a form of euvolemic, hypo‐osmolar hyponatremia, which is characterized by a negative free water clearance with high urine osmolality and low plasma osmolality in the absence of renal, adrenal, and thyroid insufficiency.131 The causes of SIADH can include malignancy (small‐cell lung cancer, lymphoma), central nervous system–related causes (infections, mass, trauma, bleed), iatrogenic (psychoactive drugs, chemotherapy), pulmonary disorders, and, rarely, genetic causes. The clinical presentation and morbidity and mortality associated with SIADH are related to severity and rapidity of onset as well as duration of hyponatremia. Clinical manifestations of hyponatremia are usually limited to the central nervous system and occur typically at sodium levels <125 mEq/L. Cardiac manifestations in hyponatremia are not common but recently TCMP has been described in patients with severe hyponatremia in the setting of subarachnoid hemorrhage, head trauma, adrenal insufficiency, thyroid dysfunction, and SIADH.131, 132, 133, 134, 135, 136, 137, 138, 139 Hyponatremia has been shown to exist in 32.1% of cases of TCMP.140 However, many of the cases demonstrating a relationship between hyponatremia and TCMP had confounding factors present such as hypothyroidism, adrenal insufficiency, and seizures. Table 5 summarizes all the cases with confirmed diagnoses of SIADH associated with TCMP.
Table 5.
Case Reports of TCMP in Patients Diagnosed With SIADH
| Authors (Year)Reference | Age/Sex | Presentation | Serum Sodium (mEq/L) | Seizure | Cardiac Enzymes | ECG | TTE | Cardiac Catheterization/Left Ventriculogram | Recovery |
|---|---|---|---|---|---|---|---|---|---|
| Jha et al (2016)139 | 55 yo/F | Vomiting, frontal headache, head injury 1 mo back | 108 | No | Elevated | T‐wave inversions in leads V1 to V6 | Reduced systolic function, EF 20%–25%, akinesis apical myocardium | Apical akinesis and ballooning, EF 25%, no coronary artery disease | Day 28: EF 50% |
| Kawano et al (2011)136 | 82 yo/M | Dyspnea, vomiting | 105 | No | Elevated | ST‐segment elevation in V1 to V5 | Akinesis of left ventricular apex | Normal coronaries, akinesis of LV apex | Day 14: normal kinesis of left ventricular apex |
| Urahama et al (2009)137 | 88 yo/F | Dyspnea, appetite loss | 119 | No | NA | ST‐segment elevation in the V3, V4, and V5 leads | NA | Normal coronaries | Follow‐up TTE NA, resolution of symptoms |
| AbouEzzeddine et al (2010)138 | 57 yo/F | Chest pain | 111 | No | Elevated | Normal | EF 35% | Normal coronaries, akinesis of the mid and apical segments, EF 35% | 1‐mo follow‐up: EF 69% |
| Worthley et al (2007)134 | 69 yo/F | Seizure, confusion | 109 | Yes | Mild elevation | Anterior ST segment elevation | NA | Normal coronaries, anteroapical and inferoapical dyskinesis | Day 12: normal LV function, no apical defect |
EF indicates ejection fraction; LV, left ventricle; NA, not applicable; SIADH, syndrome of inappropriate antidiuretic hormone secretion; TCMP, takotsubo cardiomyopathy; TTE, transthoracic echocardiogram.
The mechanism of transient cardiac dysfunction in the setting of hyponatremia remains unknown. In cases associated with seizures, catecholamine‐mediated processes may be explanatory but some cases have reported elevated dopamine and norepinephrine levels, possibly triggering TCMP by a mechanism other than a catecholamine‐mediated one.136 Animal models have suggested a role of intracellular calcium overload caused by impaired function of the myocyte membrane sodium–calcium pumps in the setting of hyponatremia, resulting in myocardial dysfunction and arrythmogenesis.141 Others have postulated a role of intracellular fluid shifts resulting in cellular edema. This is further supported by the finding of myocardial edema seen on cardiac imaging in patients with TCMP.136, 140
The reported threshold of serum sodium levels at which TCMP develops has varied in different cases. Kawano et al136 reported the occurrence of TCMP in 1 patient at a serum sodium concentration of 105 mEq/L, although no cardiac dysfunction occurred at serum sodium levels of 110 mEq/L in the reported prior hospital admission. An important variable to consider is the occurrence of seizures that may vary with the sodium concentration. Not all cases reporting TCMP with hyponatremia actually had a seizure episode. Therefore, though the severity of hyponatremia may predispose to reversible cardiac dysfunction, whether factors other than hyponatremia alone contribute to the cause in cases of SIADH is yet to be answered.
Conclusion
Although a number of case reports have described an association of TCMP with endocrine disorders, at this time it is difficult to provide a reliable estimate of how frequently these associations occur. Furthermore, the causal link between endocrine disorders and TCMP is still debatable and requires further research and a larger database. The development of TCMP registries across different countries could serve as a resource to help provide answers to these questions. Nevertheless, it seems imperative that clinicians be aware of such potential associations, given that identification of an endocrine disorder in a patient with TCMP may offer a potentially great therapeutic opportunity. Therefore, we suggest that as this association undergoes further study, clinicians evaluating patients with TCMP would be well advised to have a low threshold to screen such patients for endocrine dysfunction.
Disclosures
None.
J Am Heart Assoc. 2018;7:e009003 DOI: 10.1161/JAHA.118.009003.
References
- 1. Akashi YJ, Goldstein DS, Barbaro G, Ueyama T. Takotsubo cardiomyopathy. Circulation. 2008;118:2754–2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kurowski V, Kaiser A, von Hof K, Killermann DP, Mayer B, Hartmann F, Schunkert H, Radke PW. Apical and midventricular transient left ventricular dysfunction syndrome (tako‐tsubo cardiomyopathy) frequency, mechanisms, and prognosis. Chest. 2007;132:809–816. [DOI] [PubMed] [Google Scholar]
- 3. Schneider B, Athanasiadis A, Schwab J, Pistner W, Gottwald U, Schoeller R, Toepel W, Winter KD, Stellbrink C, Müller‐Honold T, Wegner C. Complications in the clinical course of tako‐tsubo cardiomyopathy. Int J Cardiol. 2014;176:199–205. [DOI] [PubMed] [Google Scholar]
- 4. Hurst RT, Prasad A, Askew JW, Sengupta PP, Tajik AJ. Takotsubo cardiomyopathy: a unique cardiomyopathy with variable ventricular morphology. JACC Cardiovasc Imaging. 2010;3:641–649. [DOI] [PubMed] [Google Scholar]
- 5. Stein AB, Tang XL, Guo Y, Xuan YT, Dawn B, Bolli R. Delayed adaptation of the heart to stress: late preconditioning. Stroke. 2004;35:2676–2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ueyama T, Kasamatsu K, Hano T, Yamamoto K, Tsuruo Y, Nishio I. Emotional stress induces transient left ventricular hypocontraction in the rat via activation of cardiac adrenoceptors: a possible animal model of “tako‐tsubo” cardiomyopathy. Circ J. 2002;66:712–713. [DOI] [PubMed] [Google Scholar]
- 7. Kume T, Kawamoto T, Okura H, Toyota E, Neishi Y, Watanabe N, Hayashida A, Okahashi N, Yoshimura Y, Saito K, Nezuo S. Local release of catecholamines from the hearts of patients with tako‐tsubo‐like left ventricular dysfunction. Circ J. 2008;72:106–108. [DOI] [PubMed] [Google Scholar]
- 8. Wittstein IS, Thiemann DR, Lima JA, Baughman KL, Schulman SP, Gerstenblith G, Wu KC, Rade JJ, Bivalacqua TJ, Champion HC. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med. 2005;352:539–548. [DOI] [PubMed] [Google Scholar]
- 9. Frustaci A, Loperfido F, Gentiloni N, Caldarulo M, Margante E, Russo MA. Catecholamine‐induced cardiomyopathy in multiple endocrine neoplasia: a histologic, ultrastructural, and biochemical study. Chest. 1991;99:382–385. [DOI] [PubMed] [Google Scholar]
- 10. Ueyama T, Kawabe T, Hano T, Tsuruo Y, Ueda K, Ichinose M, Kimura H, Yoshida KI. Upregulation of heme oxygenase‐1 in an animal model of takotsubo cardiomyopathy. Circ J. 2009;73:1141–1146. [DOI] [PubMed] [Google Scholar]
- 11. Pierpont GL, DeMaster EG, Cohn JN. Regional differences in adrenergic function within the left ventricle. Am J Physiol. 1984;246:824–829. [DOI] [PubMed] [Google Scholar]
- 12. Simoes MV, Marín‐Neto JA, Maciel BC. Variable regional left ventricular dysfunction in takotsubo cardiomyopathy syndrome. Echocardiography. 2007;24:893. [DOI] [PubMed] [Google Scholar]
- 13. Kawano H, Okada R, Yano K. Histological study on the distribution of autonomic nerves in the human heart. Heart Vessels. 2003;18:32–39. [DOI] [PubMed] [Google Scholar]
- 14. Cortese B, Robotti S, Puggioni E, Bianchi F, Lupi G, Brignole M. Transient left ventricular apical ballooning syndrome: all that glitters is not apical. J Cardiovasc Med. 2007;8:934–936. [DOI] [PubMed] [Google Scholar]
- 15. Brandspiegel HZ, Marinchak RA, Rials SJ, Kowey PR. A broken heart. Circulation. 1998;98:1349. [DOI] [PubMed] [Google Scholar]
- 16. Redfors B, Shao Y, Wikström J, Lyon AR, Oldfors A, Gan LM, Omerovic E. Contrast echocardiography reveals apparently normal coronary perfusion in a rat model of stress‐induced (takotsubo) cardiomyopathy. Eur Heart J Cardiovasc Imaging. 2013;15:152–157. [DOI] [PubMed] [Google Scholar]
- 17. Redfors B, Shao Y, Omerovic E. Stress‐induced cardiomyopathy (takotsubo)–broken heart and mind? Vasc Health Risk Manag. 2013;9:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Redfors B, Shao Y, Ali A, Omerovic E. Are the different patterns of stress‐induced (takotsubo) cardiomyopathy explained by regional mechanical overload and demand: supply mismatch in selected ventricular regions? Med Hypotheses. 2013;81:954–960. [DOI] [PubMed] [Google Scholar]
- 19. Alter P, Figiel JH, Rominger MB. Increased ventricular wall stress and late gadolinium enhancement in takotsubo cardiomyopathy. Int J Cardiol. 2014;172:e184–e186. [DOI] [PubMed] [Google Scholar]
- 20. Cain PA, Ahl R, Hedstrom E, Ugander M, Allansdotter‐Johnsson A, Friberg P, Arheden H. Age and gender specific normal values of left ventricular mass, volume and function for gradient echo magnetic resonance imaging: a cross sectional study. BMC Med Imaging. 2009;9:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. El Mahmoud R, Mansencal N, Pilliére R, Leyer F, Abbou N, Michaud P, Nallet O, Digne F, Lacombe P, Cattan S, Dubourg O. Prevalence and characteristics of left ventricular outflow tract obstruction in tako‐tsubo syndrome. Am Heart J. 2008;156:543–548. [DOI] [PubMed] [Google Scholar]
- 22. Kleinfeldt T, Schneider H, Akin I, Kische S, Turan RG, Nienaber CA, Ince H. Detection of FMR1‐gene in takotsubo cardiomyopathy: a new piece in the puzzle. Int J Cardiol. 2009;137:e81–e83. [DOI] [PubMed] [Google Scholar]
- 23. Sharkey SW, Maron BJ, Nelson P, Parpart M, Maron MS. Adrenergic receptor polymorphisms in patients with stress (tako‐tsubo) cardiomyopathy. J Cardiol. 2009;53:53–57. [DOI] [PubMed] [Google Scholar]
- 24. Adler JT, Meyer‐Rochow GY, Chen H, Benn DE, Robinson BG, Sippel RS, Sidhu SB. Pheochromocytoma: current approaches and future directions. Oncologist. 2008;13:779–793. [DOI] [PubMed] [Google Scholar]
- 25. Bybee KA, Kara T, Prasad A, Lerman A, Barsness GW, Wright RS, Rihal CS. Systematic review: transient left ventricular apical ballooning: a syndrome that mimics ST‐segment elevation myocardial infarction. Ann Intern Med. 2004;141:858–865. [DOI] [PubMed] [Google Scholar]
- 26. Kawai S, Kitabatake A, Tomoike H; Takotsubo Cardiomyopathy Study Group . Guidelines for diagnosis of takotsubo (ampulla) cardiomyopathy. Circ J. 2007;71:990–992. [DOI] [PubMed] [Google Scholar]
- 27. Zelinka T, Petrák O, Turková H, Holaj R, Štrauch B, Kršek M, Vrankova AB, Musil Z, Dušková J, Kubinyi J, Michalský D. High incidence of cardiovascular complications in pheochromocytoma. Horm Metab Res. 2012;44:379–384. [DOI] [PubMed] [Google Scholar]
- 28. Agrawal S, Shirani J, Garg L, Singh A, Longo S, Longo A, Fegley M, Stone L, Razavi M, Radoianu N, Nanda S. Pheochromocytoma and stress cardiomyopathy: insight into pathogenesis. World J Cardiol. 2017;9:255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Giavarini A, Chedid A, Bobrie G, Plouin PF, Hagège A, Amar L. Acute catecholamine cardiomyopathy in patients with phaeochromocytoma or functional paraganglioma. Heart. 2013;99:1438–1444. [DOI] [PubMed] [Google Scholar]
- 30. Park JH, Kim KS, Sul JY, Shin SK, Kim JH, Lee JH, Choi SW, Jeong JO, Seong IW. Prevalence and patterns of left ventricular dysfunction in patients with pheochromocytoma. J Cardiovasc Ultrasound. 2011;19:76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Coupez E, Eschalier R, Pereira B, Pierrard R, Souteyrand G, Clerfond G, Citron B, Lusson JR, Mansencal N, Motreff P. A single pathophysiological pathway in takotsubo cardiomyopathy: catecholaminergic stress. Arch Cardiovasc Dis. 2014;107:245–252. [DOI] [PubMed] [Google Scholar]
- 32. Y‐Hassan S. Clinical features and outcome of pheochromocytoma‐induced takotsubo syndrome: analysis of 80 published cases. Am J Cardiol. 2016;117:1836–1844. [DOI] [PubMed] [Google Scholar]
- 33. Agarwal V, Kant G, Hans N, Messerli FH. Takotsubo‐like cardiomyopathy in pheochromocytoma. Int J Cardiol. 2011;153:241–248. [DOI] [PubMed] [Google Scholar]
- 34. Gagnon N, Mansour S, Bitton Y, Bourdeau I. Takotsubo‐like cardiomyopathy in a large cohort of patients with pheochromocytoma and paraganglioma. Endocr Pract. 2017;23:1178–1192. [DOI] [PubMed] [Google Scholar]
- 35. Kuo BT, Choubey R, Novaro GM. Reduced estrogen in menopause may predispose women to takotsubo cardiomyopathy. Gend Med. 2010;7:71–77. [DOI] [PubMed] [Google Scholar]
- 36. Fischer M, Baessler A, Schunkert H. Renin angiotensin system and gender differences in the cardiovascular system. Cardiovasc Res. 2002;53:672–677. [DOI] [PubMed] [Google Scholar]
- 37. Ueyama T, Kasamatsu K, Hano T, Tsuruo Y, Ishikura F. Catecholamines and estrogen are involved in the pathogenesis of emotional stress‐induced acute heart attack. Ann N Y Acad Sci. 2008;1148:479–485. [DOI] [PubMed] [Google Scholar]
- 38. Ueyama T, Ishikura F, Matsuda A, Asanuma T, Ueda K, Ichinose M, Kasamatsu K, Hano T, Akasaka T, Tsuruo Y, Morimoto K. Chronic estrogen supplementation following ovariectomy improves the emotional stress‐induced cardiovascular responses by indirect action on the nervous system and by direct action on the heart. Circ J. 2007;71:565–573. [DOI] [PubMed] [Google Scholar]
- 39. Ueyama T, Hano T, Kasamatsu K, Yamamoto K, Tsuruo Y, Nishio I. Estrogen attenuates the emotional stress‐induced cardiac responses in the animal model of tako‐tsubo (ampulla) cardiomyopathy. J Cardiovasc Pharmacol. 2003;42:S117–S120. [DOI] [PubMed] [Google Scholar]
- 40. Thawornkaiwong A, Preawnim S, Wattanapermpool J. Upregulation of β 1‐adrenergic receptors in ovariectomized rat hearts. Life Sci. 2003;72:1813–1824. [DOI] [PubMed] [Google Scholar]
- 41. Chou AY, Saw J. Basis for sex‐specific expression of takotsubo cardiomyopathy, cardiac syndrome X, and spontaneous coronary artery dissection. Can J Cardiol. 2014;30:738–746. [DOI] [PubMed] [Google Scholar]
- 42. Sy F, Basraon J, Zheng H, Singh M, Richina J, Ambrose JA. Frequency of takotsubo cardiomyopathy in postmenopausal women presenting with an acute coronary syndrome. Am J Cardiol. 2013;112:479–482. [DOI] [PubMed] [Google Scholar]
- 43. Komesaroff PA, Sudhir K, Esler MD. Effects of estrogen on stress responses in women. J Clin Endocrinol Metab. 1999;84:4292–4293. [DOI] [PubMed] [Google Scholar]
- 44. Eliades M, El‐Maouche D, Choudhary C, Zinsmeister B, Burman KD. Takotsubo cardiomyopathy associated with thyrotoxicosis: a case report and review of the literature. Thyroid. 2014;24:383–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Biłan A, Ignatowicz A, Mosiewicz J, Wysokiński A. Dyspnea as a dominant clinical manifestation in a patient with takotsubo cardiomyopathy treated for chronic obstructive pulmonary disease and hyperthyroidism. Pol Arch Med Wewn. 2009;119:265–268. [PubMed] [Google Scholar]
- 46. Van de Donk NW, America YG, Zelissen PM, Hamer BJ. Takotsubo cardiomyopathy following radioiodine therapy for toxic multinodular goitre. Neth J Med. 2009;67:350–352. [PubMed] [Google Scholar]
- 47. Sakaki T, Fujioka Y, Akagami T, Masai M, Shimizu H, Sakoda T, Tsujino T, Ohyanagi M. Cardiac wall motion abnormalities observed in a patient with transient hyperthyroidism. Jpn Heart J. 2004;45:1071–1077. [DOI] [PubMed] [Google Scholar]
- 48. Hutchings DC, Adlam D, Ferreira V, Karamitsos TD, Channon KM. Takotsubo cardiomyopathy in association with endogenous and exogenous thyrotoxicosis. QJM. 2010;104:433–435. [DOI] [PubMed] [Google Scholar]
- 49. Tsao YT, Lin CS, Lin SH. Thyrotoxicosis factitia induces takotsubo cardiomyopathy in end‐stage renal disease: a pathogenetic hypothesis. Kidney Int. 2010;77:468. [DOI] [PubMed] [Google Scholar]
- 50. Kwon SA, Yang JH, Kim MK, Park SW, Kim SH, Park KH, Park WJ. A case of takotsubo cardiomyopathy in a patient with iatrogenic thyrotoxicosis. Int J Cardiol. 2010;145:e111–e113. [DOI] [PubMed] [Google Scholar]
- 51. Gundara JS, Lee JC, Ip J, Sidhu SB. Takotsubo cardiomyopathy complicating thyroidectomy for Graves’ disease. Thyroid. 2012;22:975–976. [DOI] [PubMed] [Google Scholar]
- 52. Sarullo FM, Americo L, Accardo S, Cicero S, Schicchi R, Schirò M, Castello A. Tako‐tsubo cardiomyopathy observed in a patient with sepsis and transient hyperthyroidism. Monaldi Arch Chest Dis. 2009;72:33–36. [DOI] [PubMed] [Google Scholar]
- 53. Alidjan F, Ezzhati M, Bruggeling W, van Guldener C. Takotsubo cardiomyopathy precipitated by thyrotoxicosis. Thyroid. 2010;20:1427–1428. [DOI] [PubMed] [Google Scholar]
- 54. Radhakrishnan A, Granato JE. An association between takotsubo cardiomyopathy and thyroid storm. Postgrad Med. 2009;121:126–130. [DOI] [PubMed] [Google Scholar]
- 55. Rossor AM, Pearce SH, Adams PC. Left ventricular apical ballooning (takotsubo cardiomyopathy) in thyrotoxicosis. Thyroid. 2007;17:181–182. [DOI] [PubMed] [Google Scholar]
- 56. Miyazaki S, Kamiishi T, Hosokawa N, Komura M, Konagai H, Sagai H, Takamoto T. Reversible left ventricular dysfunction “takotsubo” cardiomyopathy associated with hyperthyroidism. Jpn Heart J. 2004;45:889–894. [DOI] [PubMed] [Google Scholar]
- 57. Perkins MJ, Schachter DT. Biventricular takotsubo cardiomyopathy in graves hyperthyroidism. J Invasive Cardiol. 2014;26:E35–E36. [PubMed] [Google Scholar]
- 58. Martin CS, Ionescu LN, Barbu CG, Sirbu AE, Lambrescu IM, Lacau IS, Dimulescu DR, Fica SV. Takotsubo cardiomyopathy and transient thyrotoxicosis during combination therapy with interferon‐alpha and ribavirin for chronic hepatitis C. BMC Endocr Disord. 2014;14:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kuboyama O, Tokunaga T, Kobayashi K, Iwai T, Osaka Y, Umemoto T. A case of asymptomatic patient with hyperthyroidism documented the onset of takotsubo cardiomyopathy by Holter monitoring. Int J Cardiol. 2011;151:e82–e84. [DOI] [PubMed] [Google Scholar]
- 60. Micallef T, Gruppetta M, Cassar A, Fava S. Takotsubo cardiomyopathy and severe hypothyroidism. J Cardiovasc Med (Hagerstown). 2011;12:824–827. [DOI] [PubMed] [Google Scholar]
- 61. Dahdouh Z, Roule V, Bignon M, Grollier G. Recurrent tako tsubo related to subclinical hyperthyroidism. Rev Esp Cardiol. 2011;64:1069–1071. [DOI] [PubMed] [Google Scholar]
- 62. Wu WT, Hsu PC, Huang HL, Chen YC, Chien SC. A case of takotsubo cardiomyopathy precipitated by thyroid storm and diabetic ketoacidosis with poor prognosis. Acta Cardiol Sin. 2014;30:574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hatzakorzian R, Bui H, Schricker T, Backman SB. Broken heart syndrome triggered by an obstructive goiter not associated with thyrotoxicosis. Can J Anaesth. 2013;60:808–812. [DOI] [PubMed] [Google Scholar]
- 64. Brenes‐Salazar JA. Takotsubo cardiomyopathy associated with severe hypothyroidism in an elderly female. Heart Views. 2016;17:72–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zuhdi AS, Yaakob ZH, Sadiq MA, Ismail MD, Undok AW, Ahmad WA. Takotsubo cardiomyopathy in association with hyperthyroidism. Medicina (Kaunas). 2011;47:219–221. [PubMed] [Google Scholar]
- 66. Rueda D, Aguirre R, Contardo D, Finocchietto P, Hernandez S. Takotsubo myocardiopathy and hyperthyroidism: a case report and literature review. Am J Case Rep. 2017;18:865–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Patel K, Griffing GT, Hauptman PJ, Stolker JM. Recurrent takotsubo cardiomyopathy related to recurrent thyrotoxicosis. Tex Heart Inst J. 2016;43:152–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Al‐Salameh A, Allain J, Meimoun P, Benali T, Desailloud R. Takotsubo cardiomyopathy can occur in patients with apathetic hyperthyroidism. Thyroid. 2014;24:400–401. [DOI] [PubMed] [Google Scholar]
- 69. Murdoch D, O'callaghan W, Reda E, Niranjan S. Takotsubo cardiomyopathy associated with primary hyperthyroidism secondary to toxic multinodular goiter. Int J Angiol. 2016;25:e121–e122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Omar S, Ali E, Mazek H, Mahmood T, Soontrapa S, Surrez J. Takotsubo cardiomyopathy associated with hyperthyroidism treated with thyroidectomy. Proc (Bayl Univ Med Cent). 2015;28:194–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Gowda RM, Khan IA, Soodini G, Vasavada BC, Sacchi TJ. Acute myocardial infarction with normal coronary arteries associated with iatrogenic hyperthyroidism. Int J Cardiol. 2003;90:327–329. [DOI] [PubMed] [Google Scholar]
- 72. Klein I, Ojamaa K. Thyroid hormone and the cardiovascular system: from theory to practice. J Clin Endocrinol Metab. 1994;78:1026–1027. [DOI] [PubMed] [Google Scholar]
- 73. Klein I, Danzi S. Thyroid disease and the heart. Circulation. 2007;116:1725–1735. [DOI] [PubMed] [Google Scholar]
- 74. Polikar R, Burger AG, Scherrer U, Nicod P. The thyroid and the heart. Circulation. 1993;87:1435–1441. [DOI] [PubMed] [Google Scholar]
- 75. Levey GS. Catecholamine sensitivity, thyroid hormone and the heart: a reevaluation. Am J Med. 1971;50:413–420. [Google Scholar]
- 76. Crozatier B, Su JB, Corsin A. Species differences in myocardial beta‐adrenergic receptor regulation in response to hyperthyroidism. Circ Res. 1991;69:1234–1243. [DOI] [PubMed] [Google Scholar]
- 77. Featherstone HJ, Stewart DK. Angina in thyrotoxicosis: thyroid‐related coronary artery spasm. Arch Intern Med. 1983;143:554–555. [DOI] [PubMed] [Google Scholar]
- 78. Moliterno D, DeBold CR, Robertson RM. Case report: coronary vasospasm—relation to the hyperthyroid state. Am J Med Sci. 1992;304:38–42. [DOI] [PubMed] [Google Scholar]
- 79. Proskey AJ, Saksena F, Towne WD. Myocardial infarction associated with thyrotoxicosis. Chest. 1977;72:109–111. [DOI] [PubMed] [Google Scholar]
- 80. Aggarwal S, Papani R, Gupta V. Can thyroid break your heart? Role of thyroid in takotsubo cardiomyopathy: a single center retrospective study. Int J Cardiol. 2015;184:545–546. [DOI] [PubMed] [Google Scholar]
- 81. Madias JE. Is hypothyroidism (on levothyroxine replacement) a precipitant of takotsubo syndrome? Int J Cardiol. 2015;187:29–30. [DOI] [PubMed] [Google Scholar]
- 82. Heemstra KA, Burggraaf J, Van Der Klaauw AA, Romijn JA, Smit JW, Corssmit EP. Short‐term overt hypothyroidism induces sympathovagal imbalance in thyroidectomized differentiated thyroid carcinoma patients. Clin Endocrinol. 2010;72:417–421. [DOI] [PubMed] [Google Scholar]
- 83. Mahajan AS, Lal R, Dhanwal DK, Jain AK, Chowdhury V. Evaluation of autonomic functions in subclinical hypothyroid and hypothyroid patients. Indian J Endocrinol Metab. 2013;17:460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Gillis D, Volpe R, Daneman D. A young boy with a thyroid yo‐yo. J Pediatr Endocrinol Metab. 1998;11:467–470. [DOI] [PubMed] [Google Scholar]
- 85. Champion B, Gopinath B, Ma G, El‐Kaissi S, Wall JR. Conversion to Graves’ hyperthyroidism in a patient with hypothyroidism due to Hashimoto's thyroiditis documented by real‐time thyroid ultrasonography. Thyroid. 2008;18:1135–1137. [DOI] [PubMed] [Google Scholar]
- 86. Torres MS, Ramirez L, Simkin PH, Braverman LE, Emerson CH. Effect of various doses of recombinant human thyrotropin on the thyroid radioactive iodine uptake and serum levels of thyroid hormones and thyroglobulin in normal subjects. J Clin Endocrinol Metab. 2001;86:1660–1664. [DOI] [PubMed] [Google Scholar]
- 87. Bancos I, Hahner S, Tomlinson J, Arlt W. Diagnosis and management of adrenal insufficiency. Lancet Diabetes Endocrinol. 2015;3:216–226. [DOI] [PubMed] [Google Scholar]
- 88. Iga K, Hori K, Gen H. Deep negative T waves associated with reversible left ventricular dysfunction in acute adrenal crisis. Heart Vessels. 1992;7:107–111. [DOI] [PubMed] [Google Scholar]
- 89. Oki K, Matsuura W, Koide J, Saito Y, Ono Y, Yanagihara K, Imazu M. Ampulla cardiomyopathy associated with adrenal insufficiency and hypothyroidism. Int J Cardiol. 2006;108:391–392. [DOI] [PubMed] [Google Scholar]
- 90. Sakihara S, Kageyama K, Nigawara T, Kidani Y, Suda T. Ampulla (takotsubo) cardiomyopathy caused by secondary adrenal insufficiency in ACTH isolated deficiency. Endocr J. 2007;54:631–636. [DOI] [PubMed] [Google Scholar]
- 91. Gotyo N, Kida M, Horiuchi T, Hirata Y. Torsade de pointes associated with recurrent ampulla cardiomyopathy in a patient with idiopathic ACTH deficiency. Endocr J. 2009;56:807–815. [DOI] [PubMed] [Google Scholar]
- 92. Ukita C, Miyazaki H, Toyoda N, Kosaki A, Nishikawa M, Iwasaka T. Takotsubo cardiomyopathy during acute adrenal crisis due to isolated adrenocorticotropin deficiency. Intern Med. 2009;48:347–352. [DOI] [PubMed] [Google Scholar]
- 93. Murakami M, Matsushita N, Arai R, Takahashi N, Kawamura R, Suzuki S, Takekawa S, Iwashima F, Shibui T, Hata A, Ogawa Y. Isolated adrenocorticotropin deficiency associated with delirium and Takotsubo cardiomyopathy. Case Rep Endocrinol. 2012;2012:580481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Punnam SR, Gourineni N, Gupta V. Takotsubo cardiomyopathy in a patient with Addison disease. Int J Cardiol. 2010;144:e34–e36. [DOI] [PubMed] [Google Scholar]
- 95. Wolff B, Machill K, Schulzki I, Schumacher D, Werner D. Acute reversible cardiomyopathy with cardiogenic shock in a patient with Addisonian crisis: a case report. Int J Cardiol. 2007;116:e71–e73. [DOI] [PubMed] [Google Scholar]
- 96. Eto K, Koga T, Sakamoto A, Kawazoe N, Sadoshima S, Onoyama K. Adult reversible cardiomyopathy with pituitary adrenal insufficiency caused by empty sella—a case report. Angiology. 2000;51:319–323. [DOI] [PubMed] [Google Scholar]
- 97. Singh G, Manickam A, Sethuraman M, Rathod RC. Takotsubo cardiomyopathy in a patient with pituitary adenoma and secondary adrenal insufficiency. Indian J Crit Care Med. 2015;19:731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Barcin C, Kursaklioglu H, Kose S, Amasyali B, Isik E. Takotsubo cardiomyopathy in a patient with Addison disease: is apical ballooning always reversible? Int J Cardiol. 2010;138:e15–e17. [DOI] [PubMed] [Google Scholar]
- 99. Rao MK, Xu A, Narayanan N. Glucocorticoid modulation of protein phosphorylation and sarcoplasmic reticulum function in rat myocardium. Am J Physiol Heart Circ Physiol. 2001;281:H325–H333. [DOI] [PubMed] [Google Scholar]
- 100. Pearl JM, Nelson DP, Schwartz SM, Wagner CJ, Bauer SM, Setser EA, Duffy JY. Glucocorticoids reduce ischemia‐reperfusion‐induced myocardial apoptosis in immature hearts. Ann Thorac Surg. 2002;74:830–837. [DOI] [PubMed] [Google Scholar]
- 101. Komesaroff PA, Funder JW. Differential glucocorticoid effects on catecholamine responses to stress. Am J Physiol. 1994;266:E118–E128. [DOI] [PubMed] [Google Scholar]
- 102. Cleghorn RA. Cardiovascular failure in experimental adrenal insufficiency: a historical revival. Perspect Biol Med. 1983;27:135–155. [DOI] [PubMed] [Google Scholar]
- 103. Narayanan N, Khandelwal RL. Microsomal phosphorylase in rat heart: depletion following adrenalectomy and restoration by in vivo administration of dexamethasone. Endocrinology. 1985;117:1544–1549. [DOI] [PubMed] [Google Scholar]
- 104. Radunski UK, Lund GK, Stehning C, Schnackenburg B, Bohnen S, Adam G, Blankenberg S, Muellerleile K. CMR in patients with severe myocarditis: diagnostic value of quantitative tissue markers including extracellular volume imaging. JACC Cardiovasc Imaging. 2014;7:667–675. [DOI] [PubMed] [Google Scholar]
- 105. Hoeldtke RD, Boden G, Shuman CR. Reduced epinephrine secretion and hypoglycemia unawareness in diabetic autonomic neuropathy. Ann Intern Med. 1982;96:459–462. [DOI] [PubMed] [Google Scholar]
- 106. Hoeldtke RD, Cilmi KM. Norepinephrine secretion and production in diabetic autonomic neuropathy. J Clin Endocrinol Metab. 1984;59:246–252. [DOI] [PubMed] [Google Scholar]
- 107. Inouye KE, Chan O, Yue JTY. Effects of diabetes and recurrent hypoglycemia on the regulation of the sympathoadrenal system and hypothalamo‐pituitary‐adrenal axis. Am J Physiol Endocrinol Metab. 2005;288:E422–E429. [DOI] [PubMed] [Google Scholar]
- 108. Madias JE. Low prevalence of diabetes mellitus in patients with takotsubo syndrome: a plausible ‘protective'effect with pathophysiologic connotations. Eur Heart J Acute Cardiovasc Care. 2016;5:164–170. [DOI] [PubMed] [Google Scholar]
- 109. Yayehd K, N'kenon W, Belle L, Bataille V, Hanssen M, Leddet P, Aupetit JF, Commeau P, Filippi E, Georges JL, Albert F. Management of takotsubo cardiomyopathy in non‐academic hospitals in France: the Observational French SyndromEs of TakoTsubo (OFSETT) study. Arch Cardiovas Dis. 2016;109:4–12. [DOI] [PubMed] [Google Scholar]
- 110. Núñez‐Gil IJ, Almendro‐Delia M, Andrés M, Sionis A, Martin A, Bastante T, Córdoba‐Soriano JG, Linares JA, González Sucarrats S, Sánchez‐Grande‐Flecha A, Fabregat‐Andrés O. Secondary forms of takotsubo cardiomyopathy: a whole different prognosis. Eur Heart J Acute Cardiovasc Care. 2016;5:308–316. [DOI] [PubMed] [Google Scholar]
- 111. Rathmann W, Haastert B, Icks A, Herder C, Kolb H, Holle R, Mielck A, Meisinger C, Wichmann HE, Giani G. The diabetes epidemic in the elderly population in Western Europe: data from population‐based studies. Gesundheitswesen. 2005;67:110–114. [DOI] [PubMed] [Google Scholar]
- 112. Templin C, Ghadri JR, Diekmann J, Napp LC, Bataiosu DR, Jaguszewski M, Cammann VL, Sarcon A, Geyer V, Neumann CA, Seifert B. Clinical features and outcomes of takotsubo (stress) cardiomyopathy. N Engl J Med. 2015;373:929–938. [DOI] [PubMed] [Google Scholar]
- 113. Bill V, El‐Battrawy I, Behnes M, Baumann S, Becher T, Elmas E, Hoffmann U, Haghi D, Fastner C, Kuschyk J, Papavassiliu T. “Diabetes paradox” in takotsubo cardiomyopathy. Int J Cardiol. 2016;224:88. [DOI] [PubMed] [Google Scholar]
- 114. Stiermaier T, Eitel C, Desch S, Fuernau G, Schuler G, Thiele H, Eitel I. Incidence, determinants and prognostic relevance of cardiogenic shock in patients with takotsubo cardiomyopathy. Eur Heart J Acute Cardiovasc Care. 2016;5:489–496. [DOI] [PubMed] [Google Scholar]
- 115. Oe K, Mori K, Otsuji M, Konno T, Fujino N, Yamagishi M. Takotsubo cardiomyopathy with marked ST‐segment elevation and electrical alternans complicated with hyperglycemic hyperosmolar state. Int Heart J. 2008;49:629–635. [DOI] [PubMed] [Google Scholar]
- 116. Katayama Y, Hifumi T, Inoue J, Koido Y. A case of takotsubo cardiomyopathy induced by accidental hypothermia and diabetic ketoacidosis. BMJ Case Rep. 2013;2013:bcr2012008143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Kochanowski J, Piatkowski R, Budnik M, Jasik M, Roik M, Opolski G. Biventricular takotsubo cardiomyopathy in an elderly woman with uncontrolled type 2 diabetes: the biphasic echocardiographic and clinical pattern. Acta Diabetol. 2016;53:1061–1063. [DOI] [PubMed] [Google Scholar]
- 118. Katoh S, Yamada Y, Shinohe R, Aoki K, Abe M. Takotsubo cardiomyopathy associated with hypoglycemia: inverted takotsubo contractile pattern. Am J Emerg Med. 2012;30:2098.e1. [DOI] [PubMed] [Google Scholar]
- 119. Hsu CT, Chen CK, Chang RY, Chen YP. Inverted takotsubo cardiomyopathy caused by hypoglycemia. J Emerg Crit Care Med. 2010;21:178–179. [Google Scholar]
- 120. Ansari MJ, Prasad A, Pellikka PA, Klarich KW. Takotsubo cardiomyopathy caused by hypoglycemia: a unique association with coronary arterial calcification. Int J Cardiol. 2011;147:e21–e23. [DOI] [PubMed] [Google Scholar]
- 121. Ohwada R, Hotta M, Kimura H, Takagi S, Matsuda N, Nomura K, Takano K. Ampulla cardiomyopathy after hypoglycemia in three young female patients with anorexia nervosa. Intern Med. 2005;44:228–233. [DOI] [PubMed] [Google Scholar]
- 122. Rotondi F, Manganelli F, Lanzillo T, Candelmo F, Di Lorenzo E, Marino L, Stanco G. Tako‐tsubo cardiomyopathy complicated by recurrent torsade de pointes in a patient with anorexia nervosa. Intern Med. 2010;49:1133–1137. [DOI] [PubMed] [Google Scholar]
- 123. Volman MN, Ten Kate RW, Tukkie R. Tako tsubo cardiomyopathy, presenting with cardiogenic shock in a 24‐year‐old patient with anorexia nervosa. Neth J Med. 2011;69:129–131. [PubMed] [Google Scholar]
- 124. Neufeld M, Maclaren N, Blizzard R. Autoimmune polyglandular syndromes. Pediatr Ann. 1980;9:43–53. [PubMed] [Google Scholar]
- 125. Cutolo M. Autoimmune polyendocrine syndromes. Autoimmun Rev. 2014;13:85–89. [DOI] [PubMed] [Google Scholar]
- 126. Karavelioglu Y, Baran A, Karapinar H, Küçükdurmaz Z, Yilmaz A. Reversible cardiomyopathy associated with autoimmune polyendocrine syndrome type II. Intern Med. 2013;52:981–985. [DOI] [PubMed] [Google Scholar]
- 127. Kumar KV, Pushkaraj SG, Krishnaleela BL, Modi KD. Peripartum cardiomyopathy in type II autoimmune polyendocrine syndrome. Int J Cardiol. 2011;149:e14–e15. [DOI] [PubMed] [Google Scholar]
- 128. Nielsen TD, Steenbergen C, Russell SD. Nonischemic cardiomyopathy associated with autoimmune polyglandular syndrome type II. Endocr Pract. 2007;13:59–62. [DOI] [PubMed] [Google Scholar]
- 129. Lim T, Murakami H, Hayashi K, Watanabe H, Sasaki H, Muto H, Asano Y, Miyamoto K, Omoto Y, Yamaguchi Y, Hirokami M. Takotsubo cardiomyopathy associated with autoimmune polyendocrine syndrome II. J Cardiol. 2009;53:306–310. [DOI] [PubMed] [Google Scholar]
- 130. Yehya A, Sperling L, Jacobs S, Mashman W. Takotsubo cardiomyopathy in a patient with presumptive autoimmune polyendocrine syndrome II. Cardiac Cath Lab Dir. 2011;1:118–120. [Google Scholar]
- 131. Palmer BF. Hyponatremia in patients with central nervous system disease: SIADH versus CSW. Trends Endocrinol Metab. 2003;14:182–187. [DOI] [PubMed] [Google Scholar]
- 132. Patnaik S, Punjabi C, Nathan R, Khurram I, Witzke C, Lai YK. Bland and broken hearted: a case of hyponatremia induced tako‐tsubo cardiomyopathy. Int J Cardiol. 2015;187:267–271. [DOI] [PubMed] [Google Scholar]
- 133. Chikata A, Omi W, Saeki T, Nagai H, Sakagami S. Repeated pacemaker dysfunction in a patient with recurrent takotsubo cardiomyopathy precipitated by hyponatremia. Int J Cardiol. 2014;170:443–444. [DOI] [PubMed] [Google Scholar]
- 134. Worthley MI, Anderson TJ. Transient left ventricular apical ballooning syndrome following a hyponatraemic seizure. Int J Cardiol. 2007;115:e102–e104. [DOI] [PubMed] [Google Scholar]
- 135. Santos M, Dias V, Meireles A, Gomes C, Luz A, Mendes D, Caiado L, Carvalho H, Cabral S, Torres S. Hyponatremia–an unusual trigger of takotsubo cardiomyopathy. Rev Port Cardiol. 2011;30:845–848. [DOI] [PubMed] [Google Scholar]
- 136. Kawano H, Matsumoto Y, Arakawa S, Hayano M, Fijisawa H. Takotsubo cardiomyopathy in a patient with severe hyponatremia associated with syndrome of inappropriate antidiuretic hormone. Intern Med. 2011;50:727–732. [DOI] [PubMed] [Google Scholar]
- 137. Urahama N, Nakashima H, Kurose H, Ohno T. Takotsubo cardiomyopathy with hypervasopressinemia. J Am Geriatr Soc. 2009;57:1119–1120. [DOI] [PubMed] [Google Scholar]
- 138. AbouEzzeddine O, Prasad A. Apical ballooning syndrome precipitated hyponatremia. Int J Cardiol. 2010;145:e26–e29. [DOI] [PubMed] [Google Scholar]
- 139. Jha KK, Kumar M, Jha U, Desar S. Takotsubo cardiomyopathy in a patient with SIADH. Int J Cardiol. 2016;225:342–344. [DOI] [PubMed] [Google Scholar]
- 140. Eitel I, von Knobelsdorff‐Brenkenhoff F, Bernhardt P, Carbone I, Muellerleile K, Aldrovandi A, Francone M, Desch S, Gutberlet M, Strohm O, Schuler G. Clinical characteristics and cardiovascular magnetic resonance findings in stress (takotsubo) cardiomyopathy. JAMA. 2011;306:277–286. [DOI] [PubMed] [Google Scholar]
- 141. Kolar FR, Cole WC, Ostadal BO, Dhalla NS. Transient inotropic effects of low extracellular sodium in perfused rat heart. Am J Physiol. 1990;259:H712–H719. [DOI] [PubMed] [Google Scholar]


