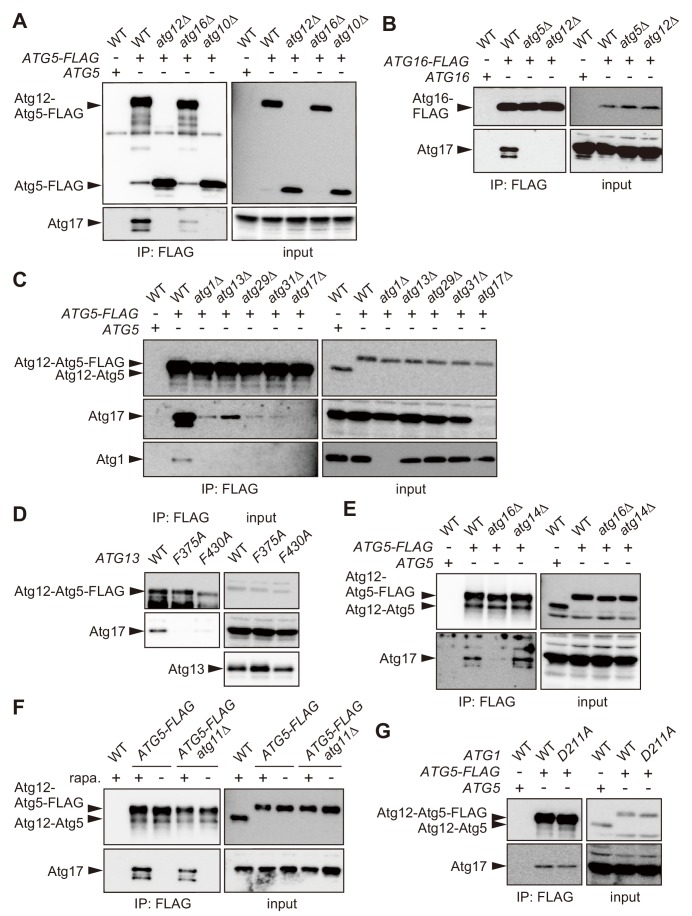
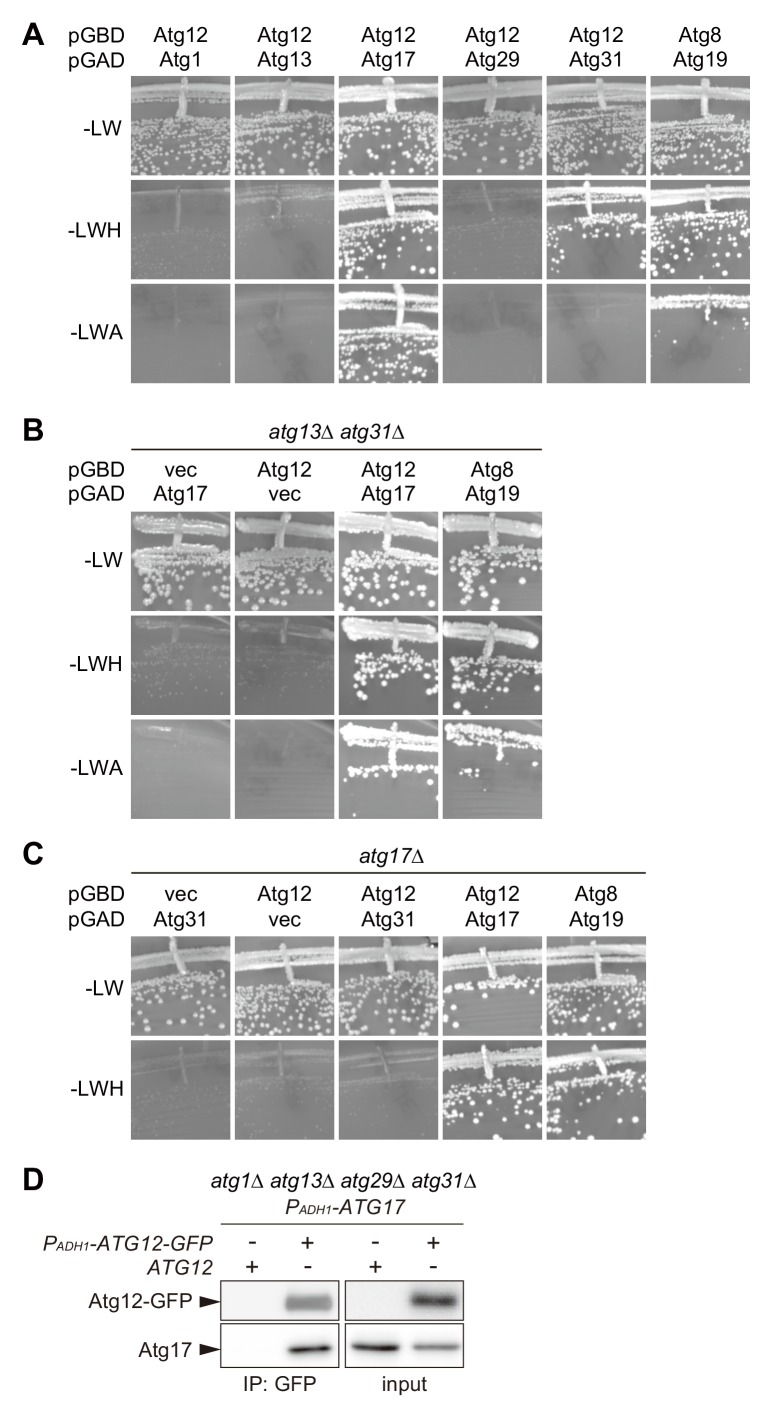
Figure 2. The Atg16 complex interacts with the Atg1 complex.
(A–C, E) Yeast cells expressing Atg5-FLAG (A, C–E) or Atg16-FLAG (B) from each chromosomal locus were treated with rapamycin for 2 hr, and subjected to immunoprecipitation using anti-FLAG antibody. The immunoprecipitates were analyzed by immunoblotting using antibodies against FLAG (A, B), Atg12 (C, E), Atg17 (A–C, E), and Atg1 (C). (D) atg13Δ cells expressing wild-type Atg13, the F375A mutant, or the F430A mutant from centromeric plasmids were treated with rapamycin for 2 hr, subjected to immunoprecipitation using anti-FLAG antibody, and the immunoprecipitates were analyzed by immunoblotting using antibodies against Atg12, Atg13 and Atg17. (F) Yeast cells were treated with or without rapamycin for 2 hr, and coimmunoprecipitation of Atg17 with Atg5-FLAG was examined as described in Figure 2C. (G) Coimmunoprecipitation of Atg17 with Atg5-FLAG was analyzed in cells expressing wild-type Atg1 or the D211A mutant from the original chromosomal locus as described in Figure 2C.